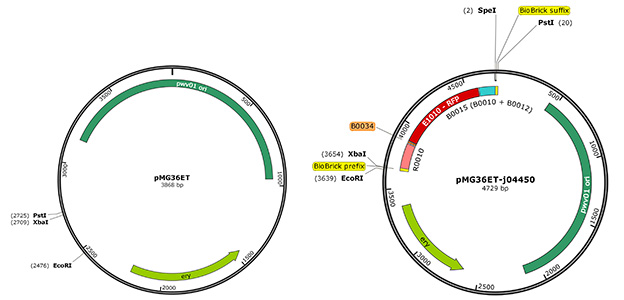
E. coli is an exceptionally easy to work with organism due to how thoroughly it has been researched – therefore, we planned to do all our BioBrick assembly in E. coli, and then transform S. thermophilus with the constructs. To easily transfer these constructs between E. coli and S. thermophilus, a BioBrick compatible shuttle vector between these two organisms had to be constructed. We utilised pMG36ET, a shuttle vector already used in previous S. thermophilus research [1]. Key features of this plasmid include a gene encoding erythromycin resistance and a pWV01 origin of replication, which has a broad host range. Due to the presence of the pWV01 origin of replication, the shuttle vector does have a very low copy number in comparison to pSB1C3. The plasmid had to be made BioBrick compatible by cutting at the highlighted EcoRI and PstI sites and inserting an oligo with the BioBrick prefix and suffix (Figure 1).
We were able to transform both E. coli and S. thermophilus with this plasmid, resulting in erythromycin-resistant colonies. By using this plasmid as a shuttle vector, we were able to clone BioBrick constructs in E. coli, and then purify the DNA for transformation of S. thermophilus. During the course of the project, we successfully transformed S. thermophilus with an amilCP BioBrick construct in this shuttle vector - therefore, we have confirmed that the plasmid is suitable for use in both E. coli and S. thermophilus.
Parts submitted to the registry
We have submitted a BioBrick compatible version of the shuttle vector to the Parts registry for future teams working with S. thermophilus (BBa_K2151666)
References
- ↑ Fontaine, L. and Hols, P. (2007). The Inhibitory Spectrum of Thermophilin 9 from Streptococcus thermophilus LMD-9 Depends on the Production of Multiple Peptides and the Activity of BlpGSt, a Thiol-Disulfide Oxidase. Applied and Environmental Microbiology, 74(4), pp.1102-1110.