Yeast epinephrine sensor-assisting phaeochromocytoma diagnosis
Background
Epinephrine and phaeochromocytoma
A pheochromocytoma or phaeochromocytoma (PCC) is a neuroendocrine tumor of the medulla of the adrenal glands (originating in the chromaffin cells), or extra-adrenal chromaffin tissue that failed to involute after birth, that secretes high amounts of catecholamines, which include norepinephrine and epinephrine. Most but not all the clinical signs and symptoms of phaeochromocytoma are due to the direct actions of secreted catecholamines. Hypertension, tachycardia, pallor, headache, and feelings of panic or anxiety, usually dominate the clinical presentation. Metabolic effects include hyperglycemia, lactic acidosis, and weight loss[1]. And early diagnosis of it can lead to regular surveillance and eventual treatment and thus improve the prognosis and patient’s life quality.
Biochemical presentation of excessive production of catecholamines is an essential step for the diagnosis of phaeochromocytoma. But as in most of the places, the measurement of epinephrine can only be carried out in hospitals, the long traveling time and high expenditure have held people back from getting physical examinations. So we picked the disease biomarker, epinephrine, as the molecule to be detected, aiming at designing a yeast-based urine epinephrine sensor for urine sample tests at home.
As with all biochemical tests of disease biomarkers, a remaining difficulty is that a positive result of high level of epinephrine does not always reliably indicate a phaeochromocytoma. So our device is to help people find out if they need to go to the hospital for further physical examination for diagnosis of phaeochromocytoma.
The catecholamine receptor: ADRB2
The beta-2 adrenergic receptor (β2 adrenoreceptor), also known as ADRB2, is a cell membrane-spanning beta-adrenergic receptor that interacts with epinephrine. It is an important GPCR and its signaling, via a downstream L-type calcium channel interaction, mediates physiologic responses such as smooth muscle relaxation and bronchodilation. The putative ligand binding region of the receptor is the outer part of the transmembrane domain[2].
Design and characterization
Design
As ADRB2 was successfully expressed in yeast Saccharomyces cerevisiae and methylotrophic yeast Pichia pastoris and successfully localized on the membrane[3, 4], we just first used PCR to amplify the fragment from human genomic DNA and cloned it downstream the yeast ADH1 promoter and GAL1 promoter then transformed the strain SMT-pAN, yielding strains SMT-pANpAA and SMT-pANpGA respectively. In order to make sure that our heterologously expressed GPCR is located on the membrane, we added His-tag at the C terminus of the one downstream ADH1 promoter (strain SMT-pANpAAhis).
Characterization
We did immunocytochemistry experiments to test the localization of the membrane protein ADRB2. The results showed that the signal of the second antibody was mainly located on the cell membrane, which indicated that heterologously expressed ADRB2 was successfully located on cell membrane (Fig.1).
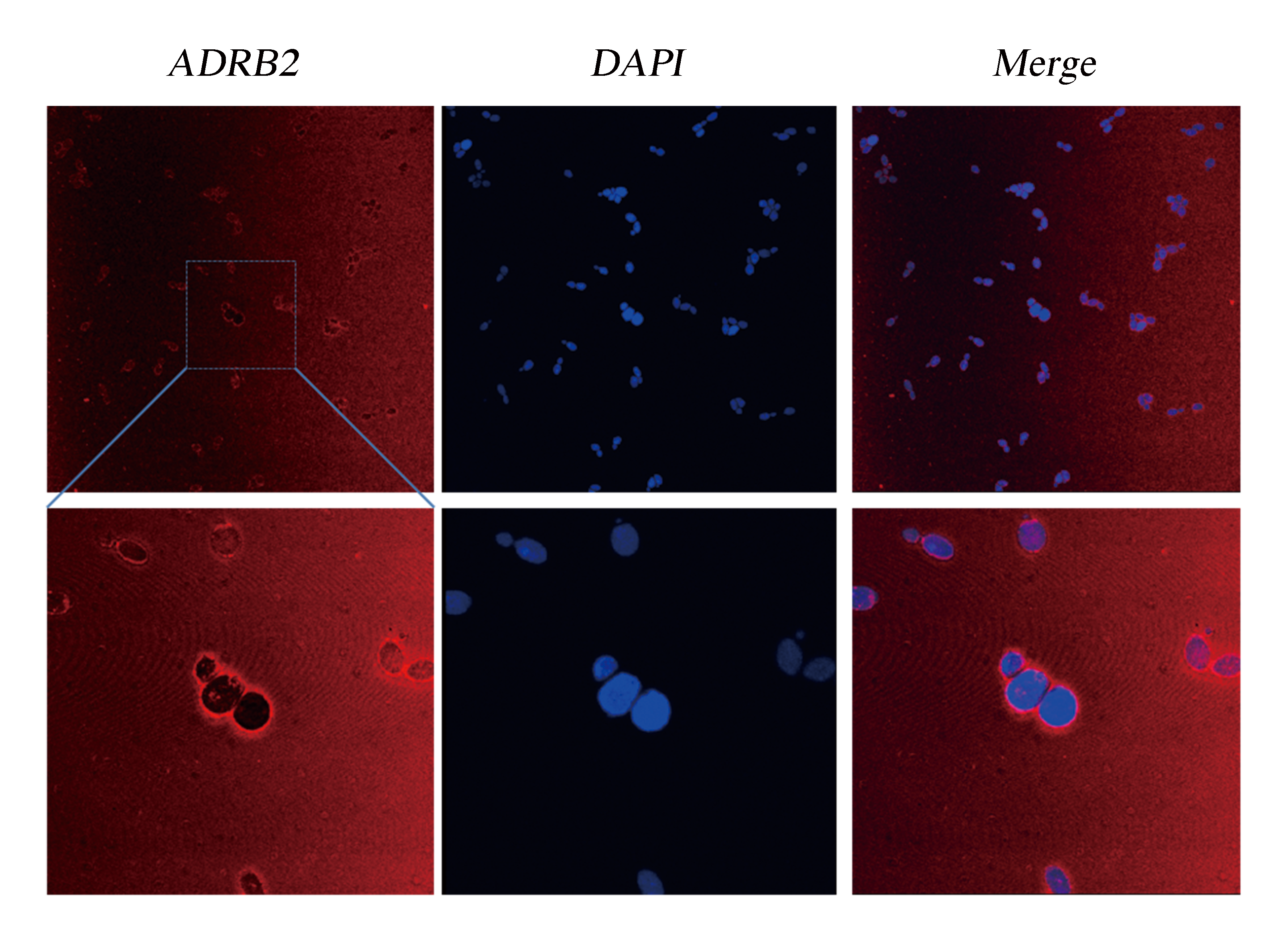
Fig.1 Detection of Membrane Localization of ADRB2 by Immunocytochemistry. The yeast cells were stabilized and stained with DAPI. As ADRB2 is expressed with a His-tag at C' terminal, thus anti-His mAb is used as the primary antibody and dunkey anti-mouse IgG 594 (Invitrogen) as the second antibody. Pictures were taken with Leica-SP5, 63 X.
Although the functional coupling of ADRB2 with yeast endogenous pheromone sensing pathway has been well studied in past few decades, the feasibility of functional signal transduction via ADRB2-Gpa2 has never been tested[4]. So we used our engineered strains, SMT-pANpAA and SMT-pANpGN, as biosensors to detect extracellular epinephrine concentration in PBS solution. The response of the epinephrine is detected within first 5 minutes after addition of epinephrine (dissolved in HAc then diluted using PBS) (Fig.2).
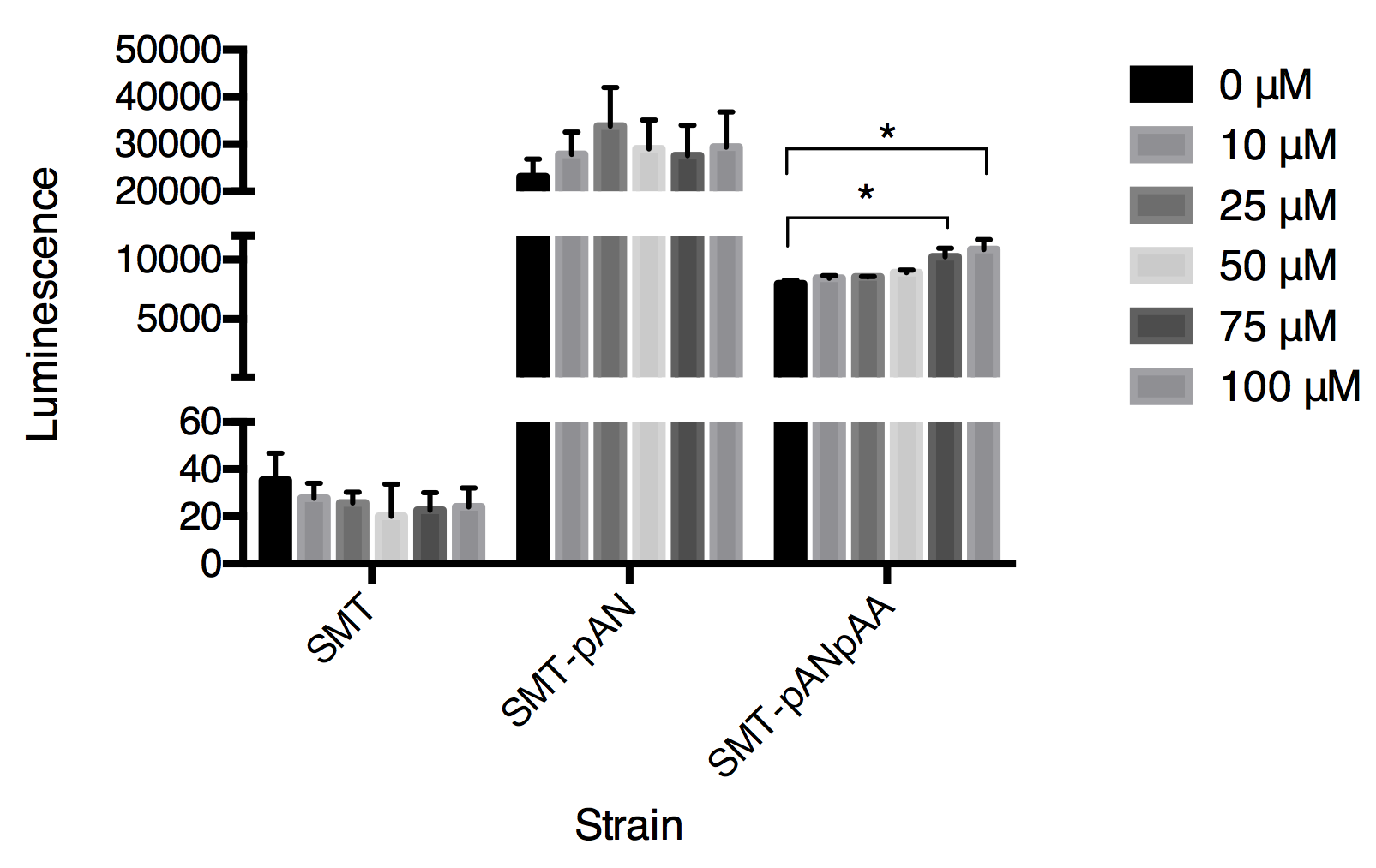
Fig.2 The luminescence triggered by epinephrine detected in three strains. Luminescence was collected at 30s after adding epinephrine. Data expressed as mean ± SD, significance was analyzed using Student-t test, n=3.
In this graph, we can tell that the constructed strain SMT-pANpAA was able to differentiate solution with relatively high concentration of epinephrine (75-100μM) with P value less than 0.05 by Student-t test. Although the difference between 100 μM and 75 μM treatment group was significant, the device can’t differentiate epinephrine solution at low concentration. And the expression of two exogenous protein under the same constitutive promoter ADH1 seems to interfere with each other as the overall luminescence decrease in strain SMT-pANpAA compared with strain SMT-pAN.
In order to figure out if the promoter usage can affect the luminescence, we tested the strain SMT- pANpGA whose biobrick part was not compatible with the official standard. Because the time was limited, we only carried out one experiment group, and unfortunately, this group didn’t help us find out the reason as it is of almost the same pattern as SMT- pANpAA (Fig.3).
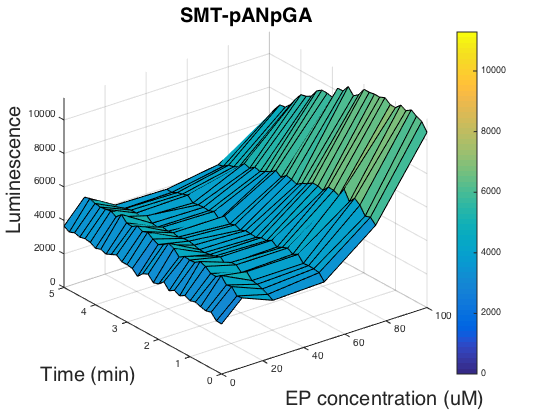
Researchers have shown that chimeric G alpha proteins of human and yeast can increase the functional coupling in heterologous expressed yeast cells[5, 6]. So we used the 5 amino acids at human G alpha protein S1 to replace the corresponding region of Gpa2, yielding biobrick BBa_K1969024, however, we didn’t go any further than finishing constructing the genomic insertion vector.
Summary
In this stage, we have:
- 1. Demonstrated the functional expression and membrane localization of ADRB2 expressed under ADH1 promoter in Saccharomyces cerevisiae.
- 2. Experimentally validated that our constructed biobrick parts BBa_K1969019 in strain SMT-pANpAA and BBa_K1969020 in strain SMT-pANpAAhis work as expected (success in detecting expression and membrane localization or differentiating epinephrine solutions with different concentrations).
In order to make these biosensor possess a long shelf life so that people can store them and use them with ease, we tried freeze-drying method to keep our contructed yeast device active-dry in our final stage of the project.
Reference
[1] Lenders, J.W.M., et al., Phaeochromocytoma. Lancet, 2005. 366(9486): p. 665-675.
[2] Rasmussen, S.G., et al., Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature, 2007. 450(7168): p. 383-7.
[3] Noguchi, S. and Y. Satow, Purification of human beta(2)-adrenergic receptor expressed in methylotrophic yeast Pichia pastoris. Journal of Biochemistry, 2006. 140(6): p. 799-804.
[4] Duport, C., J. Loeper, and A.D. Strosberg, Comparative expression of the human beta(2) and beta(3) adrenergic receptors in Saccharomyces cerevisiae. Biochimica Et Biophysica Acta-Gene Structure and Expression, 2003. 1629(1-3): p. 34-43.
[5] O'Malley, M.A., et al., Progress toward heterologous expression of active G-protein-coupled receptors in Saccharomyces cerevisiae: Linking cellular stress response with translocation and trafficking. Protein Sci, 2009. 18(11): p. 2356-70.
[6] Ishii, J., et al., Microbial fluorescence sensing for human neurotensin receptor type 1 using G alpha-engineered yeast cells. Analytical Biochemistry, 2014. 446: p. 37-43.




