Zigapusnik (Talk | contribs) |
Zigapusnik (Talk | contribs) |
||
| Line 223: | Line 223: | ||
</map> | </map> | ||
<div class="popup module1"> | <div class="popup module1"> | ||
| − | <span class="popuptext" id="module1"><b>Enhanced mechanosensing:</b> Sensitivity of mammalian cells to ultrasound or other mechanical stimuli was enhanced by the introduction of mechanosensitive ion channels and/or by the expression of protein gas vesicles from bacteria. Influx of calcium through channels is sensed by formation of a complex between calmodulin and M13 peptide that can result in a rapid light emission by cells (used for cell painting) or reconstitution of a split protease.</span> | + | <span class="popuptext" id="module1"><b>Enhanced mechanosensing:</b><br /> Sensitivity of mammalian cells to ultrasound or other mechanical stimuli was enhanced by the introduction of mechanosensitive ion channels and/or by the expression of protein gas vesicles from bacteria. Influx of calcium through channels is sensed by formation of a complex between calmodulin and M13 peptide that can result in a rapid light emission by cells (used for cell painting) or reconstitution of a split protease.</span> |
</div> | </div> | ||
<div class="popup module2"> | <div class="popup module2"> | ||
Revision as of 13:55, 15 October 2016
Sonicell
Abstract in plain English
Synthetic biology aims to control cells so they can obey our commands and do what we want, for example to produce drugs when needed. In our project we made cells respond to ultrasound or touch. When we touch the cells they light up, which can be recorded on a camera. Ideally we want cells to respond to our commands as fast as possible, because sometimes we can’t wait an hour before the cells produce the medicine and release it. That is why we gave cells a novel mechanism of processing information.
We achieved this by combining several enzymes that recognize very specific parts of proteins and cut them, which changes their function. This allowed us to combine different signals, like sound, touch, light or chemicals, to obtain the desired cell response. The new enzymes can also cut the anchor with which medicines are attached to cells after the cells make them. Among many possible uses of our inventions, we can imagine activating cells in the brain by ultrasound, which means that we don’t need to use
surgery to help people with Parkinson’s disease, or can trigger fast production of insulin in the body, to help people with diabetes.
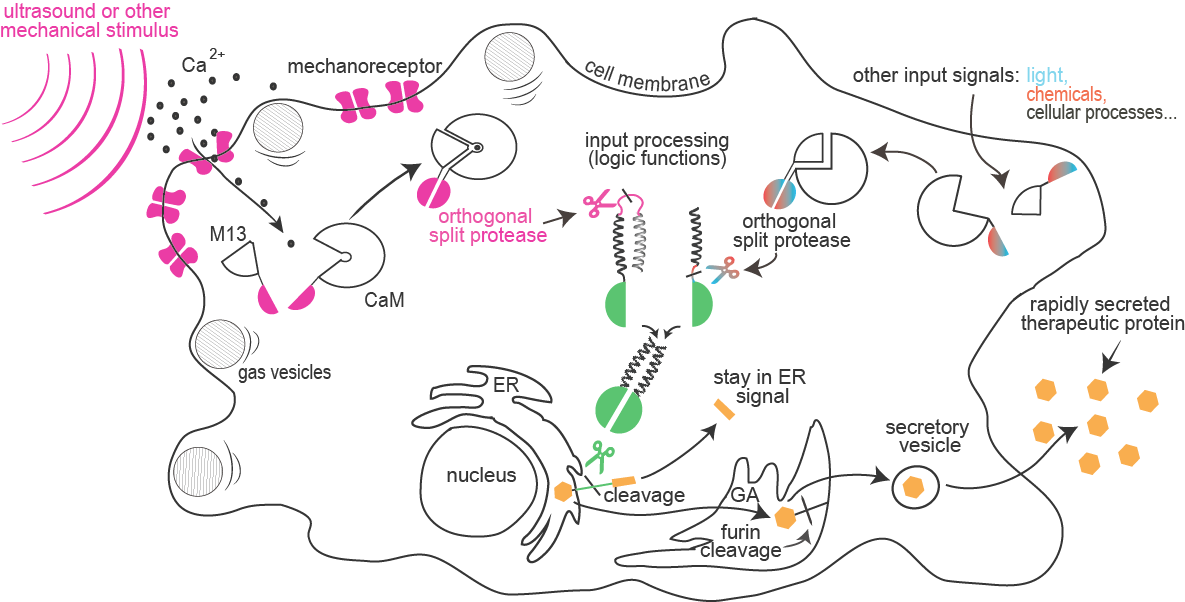
Sensitivity of mammalian cells to ultrasound or other mechanical stimuli was enhanced by the introduction of mechanosensitive ion channels and/or by the expression of protein gas vesicles from bacteria. Influx of calcium through channels is sensed by formation of a complex between calmodulin and M13 peptide that can result in a rapid light emission by cells (used for cell painting) or reconstitution of a split protease.
Combinations of proteolytic activities against specific targets resulted in activation of a reporter or another protease, which forms the basis for the design of a new type of rapid signaling pathways and construction of logic functions.
A collection of orthogonal site-specific proteases that recognize different targets was prepared as split proteins, whose activity against selected targets can be induced by stimulation with an external signal such as light or chemicals.
A rapid cellular response by secretion of a protein is triggered by the proteolytic cleavage of an endoplasmic reticulum retention peptide. After the cleavage the cargo protein is moved from the ER, and secreted as therapeutic protein.
Achievements
![]() new at science
new at science
![]() new at iGEM
new at iGEM
- Mammalian cell sensitivity to ultrasound and mechanical stimuli was increased by ectopic expression of bacterial or human cation permeable channels and functional reconstitution of bacterial protein gas vesicles from two protein components (GvpA and GvpC)

- A custom-made ultrasound generator device was used to stimulate mammalian cells

- A mechano-sensory luciferase reporter sensitive to an influx of free calcium ions was introduced into mammalian cells, which enabled rapid light emission of mammalian cells in response to mechanical stimuli and enabled painting on cells by touch with exciting potentials for other applications

- A circular proteolysis-activated luciferase reporter was experimentally verified and introduced into the iGEM collection

- A set of four different orthogonal site-specific proteases was designed and tested as split proteins in mammalian cells

- New orthogonal protease-based signaling pathways and information processing platform was designed and several logic functions based on the combination of multiple input signals were tested experimentally

- Proteolysis of the ER retention signal was introduced as the trigger for the fast release of proteins from cells aimed to enable fast therapeutic responses such as required for the release of peptide hormones, neuroactive peptides etc.




