Zigapusnik (Talk | contribs) |
Zigapusnik (Talk | contribs) |
||
| Line 139: | Line 139: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | <p><br />HEK293 cells were transfected with plasmids expressing both gas vesicle forming proteins, GvpA and GvpC. We could obtain GvpC from the Registry and added to its characterization, while the plasmid for GvpA could not be recovered and its coding sequence was synthesized using mammalian codon usage. Expression of both proteins was confirmed by the western blot (<ref>3.3.3.</ref>) and colocalization was observed by confocal microscopy (<ref>3.3.4.</ref>).</p> | ||
<div style="float:left;"> | <div style="float:left;"> | ||
<figure data-ref="3.3.3."> | <figure data-ref="3.3.3."> | ||
| Line 145: | Line 146: | ||
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| − | </div> | + | </div> |
| − | + | <div style = "float:right;"> | |
| − | + | ||
| − | <div style = "float: | + | |
<figure data-ref="3.3.4."> | <figure data-ref="3.3.4."> | ||
<img onclick="resize(this);" class="ui medium image" src="//2016.igem.org/wiki/images/f/fc/T--Slovenia--3.3.4.png"> | <img onclick="resize(this);" class="ui medium image" src="//2016.igem.org/wiki/images/f/fc/T--Slovenia--3.3.4.png"> | ||
| Line 154: | Line 153: | ||
</figure> | </figure> | ||
</div> | </div> | ||
| − | <p style = "clear: | + | <p style = "clear:both;"></p> |
<div style="clear:left; float:left;"> | <div style="clear:left; float:left;"> | ||
<figure data-ref="3.3.5."> | <figure data-ref="3.3.5."> | ||
Revision as of 00:14, 16 October 2016
nbsp; Gas vesicles
nbsp; Achievements
Addition of synthetic lipid microbubbles improved the responsiveness of cells to low-power ultrasound. Gas vesicle-forming proteins were expressed in mammalian cells where they improved sensitivity of cells to the ultrasound. Combination of the ectopic expression of mechanosensing bacterial channel MscS and gas vesicles-forming proteins sensitized cells to mechanical stimulation.
nbsp; Motivation
For activation of mechanoreceptors TRPC1 or MscS, a high-power ultrasound wave (900 Vpp) is required. Our aim was to improve responsiveness of cells to respond to the lower power of ultrasound as this would increase the selectivity, avoiding stimulation of endogenous channels and prevent cell damage. We decided to test gas-filled lipid microbubbles, since it has been reported that microbubbles can amplify the ultrasonic signal
Microbubbles are small gas-filled lipid vesicles which are used as contrast agents in medicine. Their size is in the range of micrometers. They work by resonating in an ultrasound beam, rapidly contracting and expanding in response to the pressure changes of the sound wave
nbsp; Results
(A)Schematic of a cell with an increased sensitivity to ultrasound stimulation in the presence of microbubbles. When exposed to mechanical stimuli the microbubbles contract and expand, resulting in activation of mechanosensitive channels on the cell membrane. (B) Mixed size distribution of lipid microbubbles was obtained. Stabilized lipid microbubbles were prepared by sonication and detected by microscopy.
Properties of microbubbles for example rigidity, are affected by the composition of the lipid membrane and the gas core. We prepared our lipid microbubbles from a mixture of DSPC:DSPE. Before sonication we added gas perfluorohexane (as described in the Protocols section), which facilitates compression and expansion of the microbubbles upon ultrasound stimulation (3.3.1.A). A heterogeneous mixture of microbubbles in the range from 5 to 100 µm in size were generated by this procedure (3.3.1.B). Microbubbles are most effective in the size range corresponding to the resonance frequency of the ultrasound. However care has to be taken in the applied energy to prevent cavitation, that can sonoporate cell membranes.
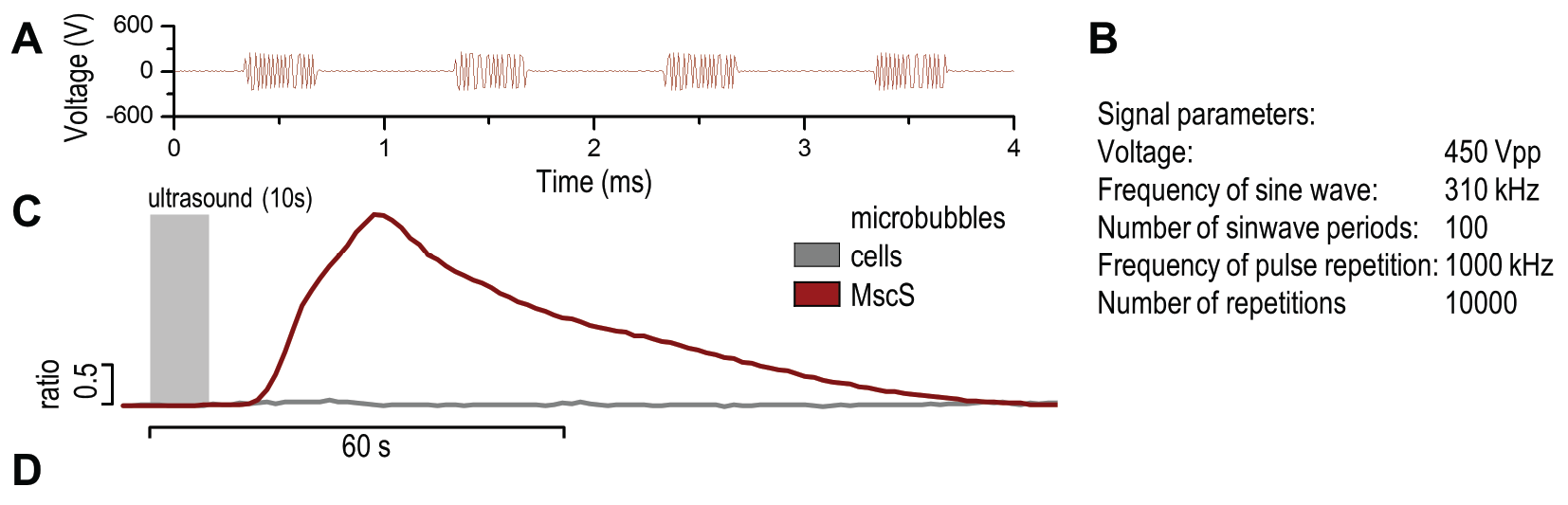
(A) Presentation of the ultrasound stimulation sequence and (B) signal parameters used for the stimulation. (C, D) Lipid microbubbles strongly enhanced response of cells expressing MscS channels. HEK293 cells expressing MscS channels were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. For comparison cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
Application of microbubbles to cells expressing mechanosensitive channel MscS significantly improved calcium influx after mechanical stimulation using low-power ultrasound wave (450 Vpp) (3.3.2.).
However, there are some drawbacks related to the use of lipid microbubbles, as their delivery requires injection into the selected tissue. Additionally lifetime of lipid microbubbles is limited in the tissue to tens of minutes and they need to be prepared freshly at least once a week.
To overcome the described drawback we thought of alternative options. One idea that initially looked too crazy to work was to use genetically encoded gas vesicles that are produced in bacteria. Bacterial gas vesicles have been used as contrasting agents for ultrasonography in animals
Gas vesicles are stable gas-filled structures, which provide buoyancy in a wide variety of planktonic prokaryotes
HEK293 cells were transfected with plasmids expressing both gas vesicle forming proteins, GvpA and GvpC. We could obtain GvpC from the Registry and added to its characterization, while the plasmid for GvpA could not be recovered and its coding sequence was synthesized using mammalian codon usage. Expression of both proteins was confirmed by the western blot (3.3.3.) and colocalization was observed by confocal microscopy (3.3.4.).
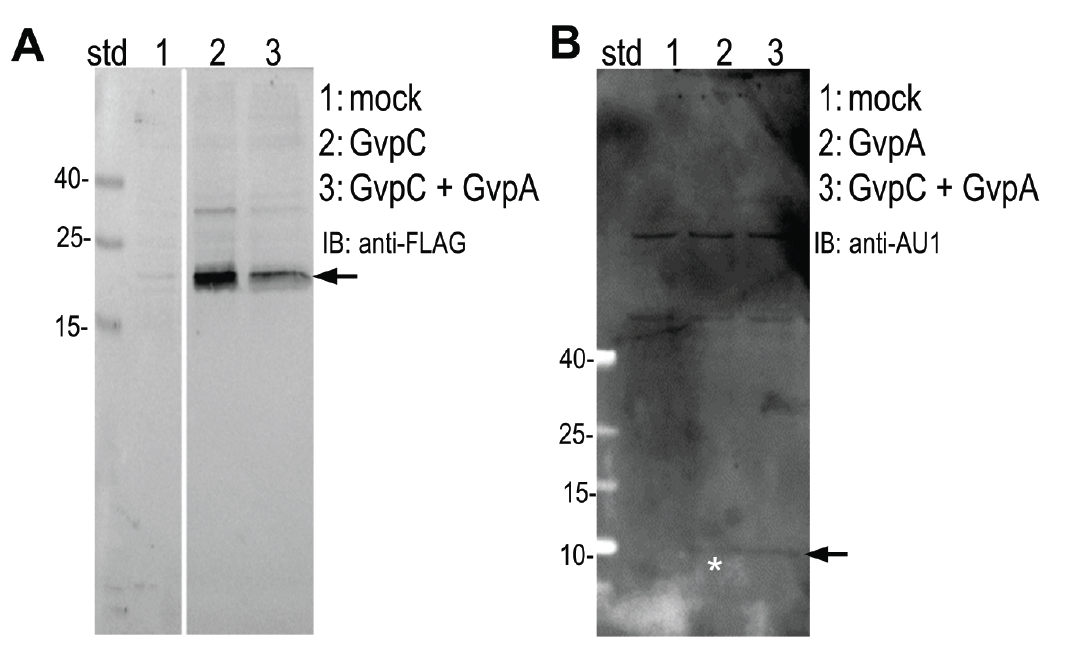
Expression of (A) GvpC and (B) GvpA protein was determined by Western blot using (A) anti-FLAG and (B) anti-AU1, respectively. Expected sizes (marked with arrow) are 22,5 kDa and 8,5 kDa for GvpC and GvpA, respectively.
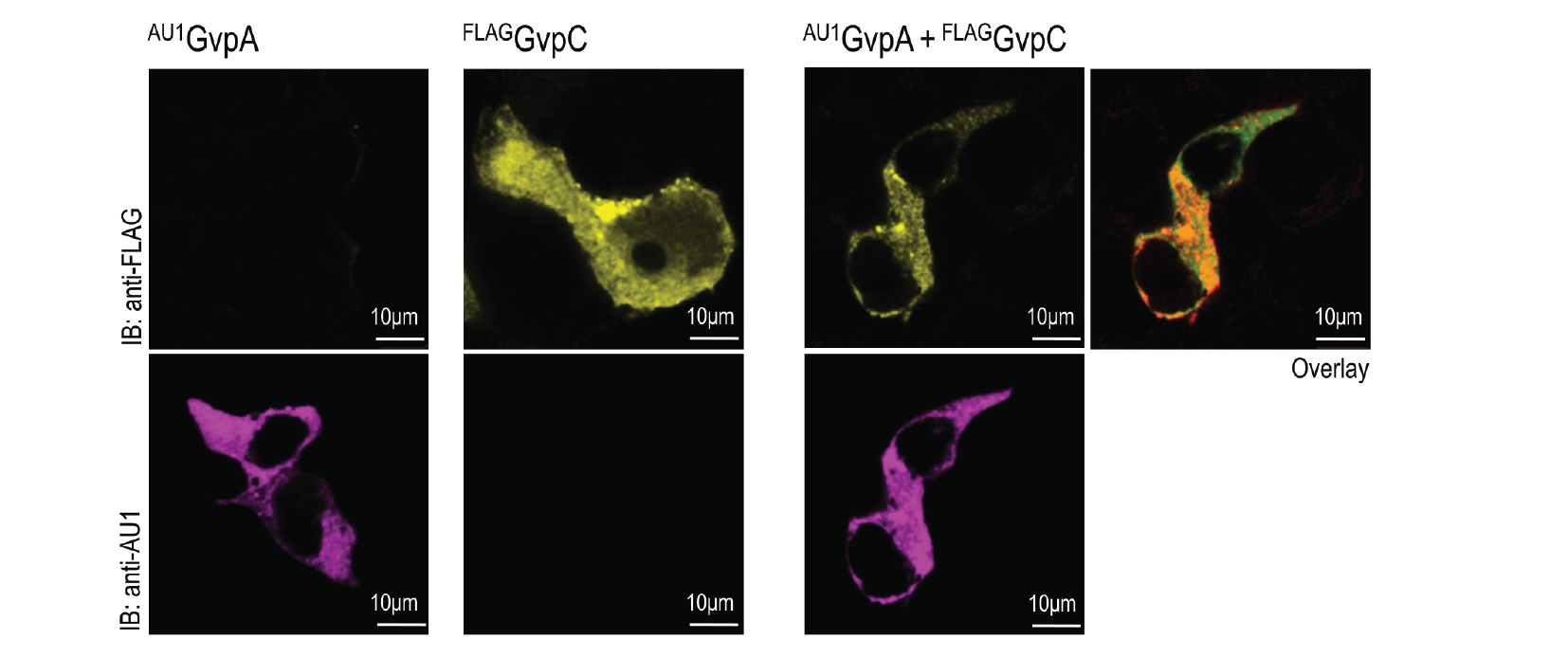
HEK293T cells were transfected with plasmids encoding gas vesicle proteins, GvpA and GvpC. 24 h after transfection cells were fixed, permeabilized and immunostained with anti-FLAG (upper row) and anti-AU1 (lower row). Both GvpA and GvpC are located in cytosol as expected. Colocalization is presented in the overlay picture.
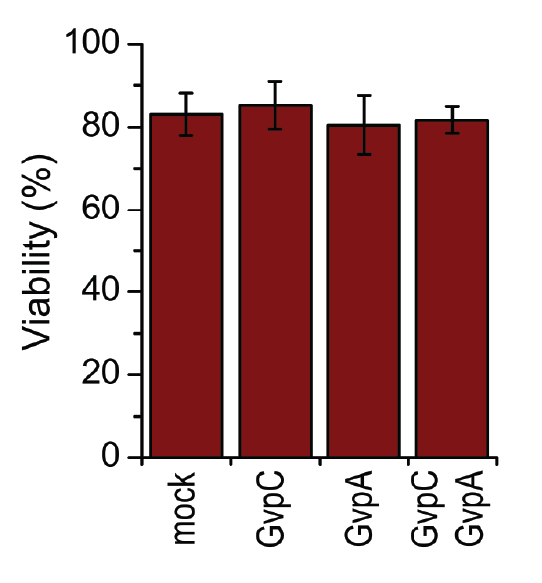
HEK293 cells were transfected with GvpA and/or GvpC. 24 h after the transfection viability of cells was measured using trypan blue.
A toxicity test was performed in order to ensure that gas vesicles were not toxic to mammalian cells. 3.3.5. shows that the viability of cells was not altered when expressing gas vesicle forming proteins.
HEK293 cells expressing gas vesicle-forming proteins exhibited increased sensitivity to ultrasound stimulation, even in the absence of exogenous mechanosensitive channels (3.3.6.), which was most likely due to activation of the endogenous mechanosensitive channels in mammalian cells.


