| (47 intermediate revisions by 4 users not shown) | |||
| Line 29: | Line 29: | ||
<b>Overview</b> | <b>Overview</b> | ||
</a> | </a> | ||
| + | <a class="item" href="//2016.igem.org/Team:Slovenia/Mechanosensing/Mechanosensitive_channels" style="color:#DB2828;"> | ||
| + | <i class="selected radio icon"></i> | ||
| + | <b>Mechanosensitive channels</b> | ||
| + | </a> | ||
<a class="item" href="#intro" style="margin-left: 10%"> | <a class="item" href="#intro" style="margin-left: 10%"> | ||
<i class="selected radio icon"></i> | <i class="selected radio icon"></i> | ||
| Line 35: | Line 39: | ||
<a class="item" href="#mot" style="margin-left: 10%"> | <a class="item" href="#mot" style="margin-left: 10%"> | ||
<i class="selected radio icon"></i> | <i class="selected radio icon"></i> | ||
| − | <b> | + | <b>Introduction</b> |
</a> | </a> | ||
<a class="item" href="#loc" style="margin-left: 10%"> | <a class="item" href="#loc" style="margin-left: 10%"> | ||
| Line 60: | Line 64: | ||
<div class="main ui citing justified container"> | <div class="main ui citing justified container"> | ||
<div> | <div> | ||
| − | <h1 class="ui left dividing header"><span id="intro" class="section"> </span>Enhanced | + | <h1 class="ui left dividing header"><span id="intro" class="section colorize"> </span>Enhanced |
| − | + | mechanosensitivity by overexpressed <br/>mechanosensitive channels</h1> | |
<div class="ui segment" style="background-color: #ebc7c7; "> | <div class="ui segment" style="background-color: #ebc7c7; "> | ||
<ul> | <ul> | ||
| Line 74: | Line 78: | ||
</div> | </div> | ||
<div class="ui segment"> | <div class="ui segment"> | ||
| − | < | + | <h3><span id="mot" class="section colorize"> </span></h3> |
| + | |||
| + | <div style="float:right; width:40%"> | ||
| + | <figure data-ref="shema"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/8/88/T--Slovenia--3.2.1.png"> | ||
| + | <figcaption><b>Proposed gating mechanisms of mechanosensitive channels (adapted from | ||
| + | Christensen & Corey | ||
| + | <x-ref>Christensen2007</x-ref> | ||
| + | ).</b></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <p> The detailed mechanism of mechanosensing is not known, however most mechanosensitive | ||
| + | receptors respond to mechanical stimuli through | ||
| + | opening of the channel pore and allowing calcium ions to enter the cell | ||
| + | <x-ref>Zheng2013</x-ref> | ||
| + | . Membrane composition has been shown to play | ||
| + | an important role in bacterial channel activation, however cytoskeleton apparently also | ||
| + | mediates mechanosensing as several mechanosensitive channels | ||
| + | comprise domains that can interact with the cytoskeleton (<ref>shema</ref>). | ||
| + | </p> | ||
| + | |||
<p>We chose to test two mechanosensitive channels: human nonspecific cation channel TRPC1 | <p>We chose to test two mechanosensitive channels: human nonspecific cation channel TRPC1 | ||
and bacterial channel MscS, previously described as important receptors involved | and bacterial channel MscS, previously described as important receptors involved | ||
| Line 81: | Line 107: | ||
. | . | ||
</p> | </p> | ||
| − | <div class="ui styled fluid accordion"> | + | <div style="width:60%" class="ui styled fluid accordion"> |
<div class="title"> | <div class="title"> | ||
<i class="dropdown icon"></i> | <i class="dropdown icon"></i> | ||
| Line 100: | Line 126: | ||
formation of functional homo- or hetero-tetramers | formation of functional homo- or hetero-tetramers | ||
<x-ref>Bianchi2007</x-ref> | <x-ref>Bianchi2007</x-ref> | ||
| − | . | + | (<ref>1a</ref>). |
</p> | </p> | ||
<div style="clear:left; width:50%"> | <div style="clear:left; width:50%"> | ||
| − | <figure data-ref=" | + | <figure data-ref="1a"> |
| − | <img onclick="resize(this);" | + | <img onclick="resize(this);" |
src="https://static.igem.org/mediawiki/2016/7/7a/T--Slovenia--3.2.2.PNG"> | src="https://static.igem.org/mediawiki/2016/7/7a/T--Slovenia--3.2.2.PNG"> | ||
<figcaption><b>Structure of a tetrameric homologous TRPV6 channel (<a | <figcaption><b>Structure of a tetrameric homologous TRPV6 channel (<a | ||
| Line 121: | Line 147: | ||
periplasm and the C-terminus embedded in the cytoplasm | periplasm and the C-terminus embedded in the cytoplasm | ||
<x-ref>Pivetti2003</x-ref> | <x-ref>Pivetti2003</x-ref> | ||
| + | (<ref>2a</ref>) | ||
. | . | ||
</p> | </p> | ||
<div style="clear:left; width:50%"> | <div style="clear:left; width:50%"> | ||
| − | <figure data-ref=" | + | <figure data-ref="2a"> |
| − | <img onclick="resize(this);" | + | <img onclick="resize(this);" |
src="https://static.igem.org/mediawiki/2016/d/df/T--Slovenia--3.2.3.PNG"> | src="https://static.igem.org/mediawiki/2016/d/df/T--Slovenia--3.2.3.PNG"> | ||
<figcaption><b>. Crystal structure of the MscS channel (<a | <figcaption><b>. Crystal structure of the MscS channel (<a | ||
| Line 135: | Line 162: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | <p style="clear:both"></p> | ||
</div> | </div> | ||
| − | <h1><span class="section"> </span>Results</h1> | + | <h1><span class="section colorize"> </span>Results</h1> |
<div class="ui segment"> | <div class="ui segment"> | ||
<div> | <div> | ||
| − | < | + | <h3><span id="loc" class="section colorize"> </span>Localization and expression</h3> |
<p>The mechanosensitive TRPC1 channel with each subunit comprising six transmembrane | <p>The mechanosensitive TRPC1 channel with each subunit comprising six transmembrane | ||
| − | helices ( | + | helices (<ref>3a</ref>A lower) and the MscS channel with three transmembrane |
| − | + | helices (<ref>3a</ref>A upper) were expressed in HEK293T cells (<ref>3a</ref>D). MscS was detected as the 31 kDa band. TRPC1 was observed at 60 kDa, which was | |
| − | + | lower than expected. We observed that the membrane localization in HEK293 was more | |
| − | helices ( | + | evident |
| − | + | for MscS (<ref>3a</ref>B) rather than TRPC1 (<ref>3a</ref>C). | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | lower than expected. We observed that the membrane localization in HEK293 was more evident | + | |
| − | for MscS ( | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</p> | </p> | ||
<div style="clear:left; width:100%"> | <div style="clear:left; width:100%"> | ||
| − | <figure data-ref=" | + | <figure data-ref="3a"> |
<img src="https://static.igem.org/mediawiki/2016/1/1e/T--Slovenia--3.2.2.png"> | <img src="https://static.igem.org/mediawiki/2016/1/1e/T--Slovenia--3.2.2.png"> | ||
<figcaption><b>Localization and expression of mechanosensitive ion channels MscS | <figcaption><b>Localization and expression of mechanosensitive ion channels MscS | ||
and TRPC1. </b><br/> | and TRPC1. </b><br/> | ||
| − | <p style="text-align:justify">(A) Scheme of bacterial ion channel MscS (upper) and human ion channel TRPC1 | + | <p style="text-align:justify">(A) Scheme of bacterial ion channel MscS |
| − | + | (upper) and human ion channel TRPC1 | |
| − | + | (lower). | |
| − | + | (B) Ion channel MscS localized to plasma membrane. (C) TRPC1 | |
| − | + | predominantly | |
| − | + | localized in the ER. | |
| − | + | (D) Ion channels MscS and TRPC1 were expressed in HEK293 cells. HEK293 | |
| − | + | cells | |
| − | + | were transfected with plasmids encoding HA tagged MscS or Myc-tagged | |
| − | + | TRPC1. | |
| + | Expression by Western blot and localization by confocal microscopy were | ||
| + | analyzed using anti-HA and anti-Myc antibodies, respectively. | ||
| + | </p> | ||
| + | </figcaption> | ||
</figure> | </figure> | ||
</div> | </div> | ||
<p style="clear:both">To improve membrane localization of TRPC1 we fused a FAS | <p style="clear:both">To improve membrane localization of TRPC1 we fused a FAS | ||
| − | transmembrane domain to TRPC1 ( | + | transmembrane domain to TRPC1 (<ref>4a</ref>A), since the |
| − | + | ||
| − | + | ||
transmembrane FAS domain has been very efficient in Jerala lab for the membrane | transmembrane FAS domain has been very efficient in Jerala lab for the membrane | ||
localization | localization | ||
| Line 188: | Line 209: | ||
of non-permeabilized cells we verified that the modified channel was inserted into | of non-permeabilized cells we verified that the modified channel was inserted into | ||
plasma membrane as predicted, since in non-permeabilized cells antibodies | plasma membrane as predicted, since in non-permeabilized cells antibodies | ||
| − | stained the exposed extracellular HA-tag but not the intracellular Myc-tag ( | + | stained the exposed extracellular HA-tag but not the intracellular Myc-tag (<ref>4a</ref>B). |
| − | + | ||
| − | + | ||
</p> | </p> | ||
<div style="float:left; width:100%"> | <div style="float:left; width:100%"> | ||
| − | <figure data-ref=" | + | <figure data-ref="4a"> |
<img src="https://static.igem.org/mediawiki/2016/1/13/T--Slovenia--3.2.3.png"> | <img src="https://static.igem.org/mediawiki/2016/1/13/T--Slovenia--3.2.3.png"> | ||
<figcaption><b>Localization of fusion protein P3:FAStm:TRPC1.</b><br/> | <figcaption><b>Localization of fusion protein P3:FAStm:TRPC1.</b><br/> | ||
| − | <p style="text-align:justify">(A) Scheme of ion channel P3:FAStm:TRPC1. (B) Ion channel P3:FAStm:TRPC1 was | + | <p style="text-align:justify">(A) Scheme of ion channel P3:FAStm:TRPC1. (B) |
| − | + | Ion channel P3:FAStm:TRPC1 was | |
| − | + | localized to plasma membrane. HEK293 cells were transfected with | |
| − | + | P3:FAStm:TRPC1 plasmid. 24 h after transfection cells were permeabilized | |
| − | + | (upper) or non-permeabilized (lower) and stained with antibodies against | |
| − | + | HA and Myc-tag. Localization on plasma membrane is shown with arrows. | |
| + | </p> | ||
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 212: | Line 232: | ||
the protein are orientated towards the interior of the cell. By addition | the protein are orientated towards the interior of the cell. By addition | ||
of the FAS transmembrane domain, the N-terminus of P3:FAStm:TRPC1 chimera (where P3 | of the FAS transmembrane domain, the N-terminus of P3:FAStm:TRPC1 chimera (where P3 | ||
| − | stands for <a href = "https://2016.igem.org/Team:Slovenia/Protease_signaling/Logic#ant">coiled coil</a>) is exposed in the extracellular | + | stands for <a |
| + | href="https://2016.igem.org/Team:Slovenia/Protease_signaling/Logic#ant">coiled | ||
| + | coil</a>) is exposed in the extracellular | ||
space and could interact with different proteins from outside the cell via the | space and could interact with different proteins from outside the cell via the | ||
N-terminal tag. We reasoned that this interaction could be used to achieve a higher | N-terminal tag. We reasoned that this interaction could be used to achieve a higher | ||
| Line 223: | Line 245: | ||
</div> | </div> | ||
<div> | <div> | ||
| − | < | + | <h3><span id="us" class="section colorize"> </span>Ultrasound stimulation</h3><br/> |
<div style="clear:both" class="ui styled fluid accordion"> | <div style="clear:both" class="ui styled fluid accordion"> | ||
<div class="title"> | <div class="title"> | ||
| Line 233: | Line 255: | ||
Ultrasound stimulation offers potentially remarkable advantages over the | Ultrasound stimulation offers potentially remarkable advantages over the | ||
majority of external stimuli used for targeted cell stimulation. | majority of external stimuli used for targeted cell stimulation. | ||
| − | Optogenetics as another | + | Optogenetics, as another |
| − | promising approach to cell stimulation requires invasive surgery to | + | promising approach to cell stimulation, requires invasive surgery to |
implement optical fibers connected to the source of light – LED or laser | implement optical fibers connected to the source of light – LED or laser | ||
<x-ref>Warden2014</x-ref> | <x-ref>Warden2014</x-ref> | ||
| Line 246: | Line 268: | ||
Previously, ultrasound had been used in several in vitro studies to directly | Previously, ultrasound had been used in several in vitro studies to directly | ||
stimulate clusters of neurons but also in few model organisms (among others | stimulate clusters of neurons but also in few model organisms (among others | ||
| − | <x-ref>King2013</x-ref> | + | <x-ref>King2013</x-ref>) |
. | . | ||
</p> | </p> | ||
| Line 253: | Line 275: | ||
<br/> | <br/> | ||
<p>For mechanical stimulation of cells with ultrasound we designed our own unique | <p>For mechanical stimulation of cells with ultrasound we designed our own unique | ||
| − | experimental setup, which included the | + | <a href="https://2016.igem.org/Team:Slovenia/Hardware#set">experimental setup</a>, |
| + | which included the | ||
<a href="https://2016.igem.org/Team:Slovenia/Hardware">ultrasound device MODUSON</a> | <a href="https://2016.igem.org/Team:Slovenia/Hardware">ultrasound device MODUSON</a> | ||
| − | that we constructed connected to the unfocused transducer Olympus | + | that we constructed and connected to the unfocused transducer Olympus |
V318-SU and a 3D printed support for a transducer to fix it at a defined position | V318-SU and a 3D printed support for a transducer to fix it at a defined position | ||
relative to the cells. Stimulation conditions were optimized for our cell | relative to the cells. Stimulation conditions were optimized for our cell | ||
| Line 263: | Line 286: | ||
concentration as a ratio of the fluorescence intensity at two wavelengths, | concentration as a ratio of the fluorescence intensity at two wavelengths, | ||
which was superior to the intensity based measurements, since it is independent of | which was superior to the intensity based measurements, since it is independent of | ||
| − | photobleaching and dye sequestration | + | photobleaching and dye sequestration. |
</p> | </p> | ||
| Line 295: | Line 318: | ||
</p> | </p> | ||
| − | <p>We showed that by expressing the MscS channel | + | <p>We showed that by expressing the MscS channel cells gained sensitivity for |
| − | ultrasound stimulation in comparison to non-transfected cells ( | + | ultrasound stimulation in comparison to non-transfected cells (<ref>5a</ref> and <ref>6a</ref>). |
| − | + | ||
| − | + | ||
Influx of calcium ions was observed at a lower rate in the case of ectopically | Influx of calcium ions was observed at a lower rate in the case of ectopically | ||
expressed TRPC1 (data not shown), probably due to its poor membrane localization. | expressed TRPC1 (data not shown), probably due to its poor membrane localization. | ||
| Line 304: | Line 325: | ||
<div style="clear:both; width:70%; margin-left:auto; margin-right:auto;"> | <div style="clear:both; width:70%; margin-left:auto; margin-right:auto;"> | ||
| − | <figure data-ref=" | + | <figure data-ref="5a"> |
<img src=" https://static.igem.org/mediawiki/2016/f/f2/T--Slovenia--S.3.1.1.png "> | <img src=" https://static.igem.org/mediawiki/2016/f/f2/T--Slovenia--S.3.1.1.png "> | ||
| − | <figcaption><b> | + | <figcaption><b>Cells expressing exogenous MscS channels gained sensitivity |
| + | for ultrasound stimulation.</b><br/> | ||
| + | <p style="text-align:justify"> | ||
| + | Scheme of cells expressing MscS. Upon stimulation with ultrasound channels open and calcium ions enter the cells. | ||
| + | </p> | ||
| + | </figcaption> | ||
</figure> | </figure> | ||
</div> | </div> | ||
<div style="clear:both;" align="center"> | <div style="clear:both;" align="center"> | ||
| − | <figure data-ref=" | + | <figure data-ref="6a"> |
<img class="ui big centered image" | <img class="ui big centered image" | ||
src="https://static.igem.org/mediawiki/2016/2/27/T--Slovenia--3.2.5.png"> | src="https://static.igem.org/mediawiki/2016/2/27/T--Slovenia--3.2.5.png"> | ||
| Line 346: | Line 372: | ||
<figcaption><b> MscS channel improves sensitivity of cells for | <figcaption><b> MscS channel improves sensitivity of cells for | ||
ultrasound.</b><br/> | ultrasound.</b><br/> | ||
| − | <p style="text-align:justify">(A) Schematic representation of a stimulation sequence and (B) signal | + | <p style="text-align:justify">(A) Schematic representation of a stimulation |
| − | + | sequence and (B) signal | |
| − | + | parameters used for stimulation. | |
| − | + | (C) and (D)Cells expressing MscS showed increased sensitivity to | |
| − | + | ultrasound | |
| − | + | stimulation in comparison to the cells without exogenous | |
| − | + | mechanosensitive | |
| − | + | channel. HEK293 cells expressing MscS channels or control cells | |
| − | + | transfected | |
| − | + | with vector were stimulated with ultrasound for 10 s and calcium influx | |
| − | + | was recorded in real time (D) using a confocal microscope. For | |
| − | + | comparison | |
| − | + | cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura | |
| + | Red | ||
| + | dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio | ||
| + | (blue line) was calculated from fluorescence intensities of Fura Red and | ||
| + | Fluo-4 | ||
| + | using <a href="https://2016.igem.org/Team:Slovenia/Software">CaPTURE</a>. | ||
| + | </p> | ||
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 365: | Line 397: | ||
improve its membrane localization, but also significantly enhanced its sensitivity | improve its membrane localization, but also significantly enhanced its sensitivity | ||
to ultrasound | to ultrasound | ||
| − | stimulation ( | + | stimulation (<ref>7a</ref> and <ref>8a</ref>), suggesting the importance of membrane localization in the function of |
| − | + | ||
| − | + | ||
mechanosensors. | mechanosensors. | ||
</p> | </p> | ||
<div style="clear:left; width:70%; margin-left:auto; margin-right:auto;"> | <div style="clear:left; width:70%; margin-left:auto; margin-right:auto;"> | ||
| − | <figure data-ref=" | + | <figure id="krneki" data-ref="7a"> |
| − | <img | + | <img |
| − | <figcaption><b> | + | src=" https://static.igem.org/mediawiki/2016/f/fd/T--Slovenia--S.3.1.2.png"> |
| + | <figcaption><b>With fusion of FAS transmembrane domain to TRPC1 we enhanced | ||
| + | sensitivity of cells to ultrasound stimulation</b><br/> | ||
| + | <p style="text-align:justify"> | ||
| + | Scheme of cells expressing modified TRPC1. Upon stimulation with ultrasound channels open and calcium ions enter the cells. | ||
| + | </p> | ||
| + | </figcaption> | ||
</figure> | </figure> | ||
</div> | </div> | ||
<div style="clear:both;" align="center"> | <div style="clear:both;" align="center"> | ||
| − | <figure data-ref=" | + | <figure data-ref="8a"> |
<img class="ui big centered image" | <img class="ui big centered image" | ||
src="https://static.igem.org/mediawiki/2016/8/8a/T--Slovenia--3.2.4.png"> | src="https://static.igem.org/mediawiki/2016/8/8a/T--Slovenia--3.2.4.png"> | ||
| Line 414: | Line 450: | ||
<figcaption><b>P3:FAS:TRPC1 channel improves sensitivity of cells for | <figcaption><b>P3:FAS:TRPC1 channel improves sensitivity of cells for | ||
ultrasound.</b><br/> | ultrasound.</b><br/> | ||
| − | <p style="text-align:justify">(A) Schematic presentation of a stimulation sequence and (B) signal | + | <p style="text-align:justify">(A) Schematic presentation of a stimulation |
| − | + | sequence and (B) signal | |
| − | + | parameters used for stimulation. | |
| − | + | (C) and (D) Cells expressing P3:FAS:TRPC1 showed increased sensitivity | |
| − | + | to | |
| − | + | ultrasound stimulation in comparison to the cells without exogenous | |
| − | + | mechanosensitive channel. | |
| − | + | HEK293 cells expressing P3:FAS:TRPC1 were stimulated with ultrasound for | |
| − | + | 10 | |
| − | + | s and calcium influx was recorded in real time (D) using a confocal | |
| − | + | microscope. For comparison | |
| − | + | cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura | |
| − | + | Red | |
| − | + | dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio | |
| + | (blue line) was calculated | ||
| + | from fluorescence intensities of Fura Red and Fluo-4 using <a | ||
| + | href="https://2016.igem.org/Team:Slovenia/Software">CaPTURE</a>. | ||
| + | </p> | ||
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
</div> | </div> | ||
<p style="clear:both">In order to observe mechanostimulation of cells with ectopically | <p style="clear:both">In order to observe mechanostimulation of cells with ectopically | ||
| − | expressed mechanoreceptors we had to use high-power ultrasound, | + | expressed mechanoreceptors we had to use high-power ultrasound. However, we tested |
that the cells nevertheless | that the cells nevertheless | ||
did not lose the viability by ultrasound stimulation. Our next challenge was to | did not lose the viability by ultrasound stimulation. Our next challenge was to | ||
| Line 441: | Line 481: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | <h3 class="ui left dividing header"><span id="ref-title" class="section colorize"> </span>References | ||
| + | </h3> | ||
| + | <div class="ui segment citing" id="references"></div> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 486: | Line 529: | ||
</script> | </script> | ||
<div> | <div> | ||
| − | + | <a href="//igem.org/Main_Page"> | |
| − | + | <img border="0" alt="iGEM" src="//2016.igem.org/wiki/images/8/84/T--Slovenia--logo_250x250.png" width="5%" | |
| − | + | style="position: fixed; bottom:0%; right:1%;"> | |
| − | </div> | + | </a> |
| + | </div> | ||
</body> | </body> | ||
</html> | </html> | ||
Latest revision as of 13:25, 19 October 2016
Enhanced
mechanosensitivity by overexpressed
mechanosensitive channels
- Ectopically expressed mechanosensitive ion channels MscS and P3:FAStm:TRPC1 were used to enhance sensitivity of mammalian cells to ultrasound stimulation.
- Membrane localization of the mechanosensitive channel TRPC1 was improved by fusing it with a FAS transmembrane domain, which also led to increased sensitivity to ultrasound stimulation.

The detailed mechanism of mechanosensing is not known, however most mechanosensitive
receptors respond to mechanical stimuli through
opening of the channel pore and allowing calcium ions to enter the cell
We chose to test two mechanosensitive channels: human nonspecific cation channel TRPC1
and bacterial channel MscS, previously described as important receptors involved
in the response to mechanical stimulation in humans and bacteria
Transient receptor potential channel 1 (TRPC1) is a human non-specific cation
channel located at the plasma membrane. It has been previously reported as
broadly expressed in human tissues where it functions as a store-operating
calcium channel

The second channel that we selected is the bacterial mechanosensitive channel
MscS. Its role is to mediate turgor regulation in bacteria and is
activated by changes in osmotic pressure

Results
Localization and expression
The mechanosensitive TRPC1 channel with each subunit comprising six transmembrane helices (3aA lower) and the MscS channel with three transmembrane helices (3aA upper) were expressed in HEK293T cells (3aD). MscS was detected as the 31 kDa band. TRPC1 was observed at 60 kDa, which was lower than expected. We observed that the membrane localization in HEK293 was more evident for MscS (3aB) rather than TRPC1 (3aC).

(A) Scheme of bacterial ion channel MscS (upper) and human ion channel TRPC1 (lower). (B) Ion channel MscS localized to plasma membrane. (C) TRPC1 predominantly localized in the ER. (D) Ion channels MscS and TRPC1 were expressed in HEK293 cells. HEK293 cells were transfected with plasmids encoding HA tagged MscS or Myc-tagged TRPC1. Expression by Western blot and localization by confocal microscopy were analyzed using anti-HA and anti-Myc antibodies, respectively.
To improve membrane localization of TRPC1 we fused a FAS
transmembrane domain to TRPC1 (4aA), since the
transmembrane FAS domain has been very efficient in Jerala lab for the membrane
localization

(A) Scheme of ion channel P3:FAStm:TRPC1. (B) Ion channel P3:FAStm:TRPC1 was localized to plasma membrane. HEK293 cells were transfected with P3:FAStm:TRPC1 plasmid. 24 h after transfection cells were permeabilized (upper) or non-permeabilized (lower) and stained with antibodies against HA and Myc-tag. Localization on plasma membrane is shown with arrows.
In addition to the improved membrane localization, the FAS transmembrane domain linked to the TRPC1 presents another advantage. The TRPC1 is an ion channel with six transmembrane helices, therefore both the N- and the C-terminus of the protein are orientated towards the interior of the cell. By addition of the FAS transmembrane domain, the N-terminus of P3:FAStm:TRPC1 chimera (where P3 stands for coiled coil) is exposed in the extracellular space and could interact with different proteins from outside the cell via the N-terminal tag. We reasoned that this interaction could be used to achieve a higher sensitivity to mechanical stimuli.
After we showed that the selected ion channels MscS, TRPC1 and P3:FAStm:TRPC1 are expressed in HEK293 and localized at the plasma membrane, we further tested their function as mechanosensors by exposing them to ultrasound stimulation.
Ultrasound stimulation
Ultrasound stimulation offers potentially remarkable advantages over the
majority of external stimuli used for targeted cell stimulation.
Optogenetics, as another
promising approach to cell stimulation, requires invasive surgery to
implement optical fibers connected to the source of light – LED or laser
For mechanical stimulation of cells with ultrasound we designed our own unique experimental setup, which included the ultrasound device MODUSON that we constructed and connected to the unfocused transducer Olympus V318-SU and a 3D printed support for a transducer to fix it at a defined position relative to the cells. Stimulation conditions were optimized for our cell line and experimental setup. To measure the changes of free calcium ion concentration we stained cells with two fluorescent dyes Fura Red and Fluo-4. The combination of these two dyes enabled us to present changes in the calcium ion concentration as a ratio of the fluorescence intensity at two wavelengths, which was superior to the intensity based measurements, since it is independent of photobleaching and dye sequestration.
Fura Red and Fluo-4 are visible light-excitable dyes used for ratiometric measurement of calcium ions which excitation maximum is at 488 nm. While Fluo-4 exhibits an increase in fluorescence emission at 515 nm upon binding of calcium ions, fluorescence emission at 655 nm of Fura Red decreases once the indicator binds calcium ions. By calculating the ratio of fluorescence emission intensities captured at 488 nm exaction (where the difference of fluorescence between the bound and free indicator is at its maximum), we could observe changes in intracellular calcium concentrations in real time.
We followed changes of calcium concentration after ultrasound stimulation in real time using ratiometric confocal microscopy. For processing of data we developed our software CaPTURE, which automatically calculated the ratio between fluorescence intensities of FuraRed and Fluo-4 and presented the data as image and calculated values.
We showed that by expressing the MscS channel cells gained sensitivity for ultrasound stimulation in comparison to non-transfected cells (5a and 6a). Influx of calcium ions was observed at a lower rate in the case of ectopically expressed TRPC1 (data not shown), probably due to its poor membrane localization.

Scheme of cells expressing MscS. Upon stimulation with ultrasound channels open and calcium ions enter the cells.

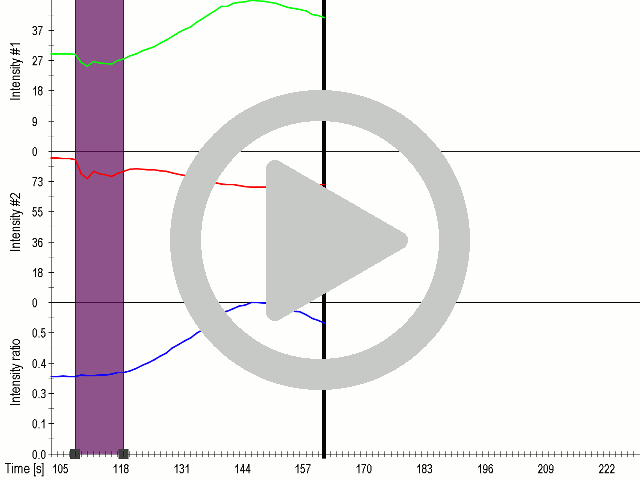

(A) Schematic representation of a stimulation sequence and (B) signal parameters used for stimulation. (C) and (D)Cells expressing MscS showed increased sensitivity to ultrasound stimulation in comparison to the cells without exogenous mechanosensitive channel. HEK293 cells expressing MscS channels or control cells transfected with vector were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. For comparison cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
Fusion of the FAS transmembrane domain to TRPC1 did not only improve its membrane localization, but also significantly enhanced its sensitivity to ultrasound stimulation (7a and 8a), suggesting the importance of membrane localization in the function of mechanosensors.

Scheme of cells expressing modified TRPC1. Upon stimulation with ultrasound channels open and calcium ions enter the cells.

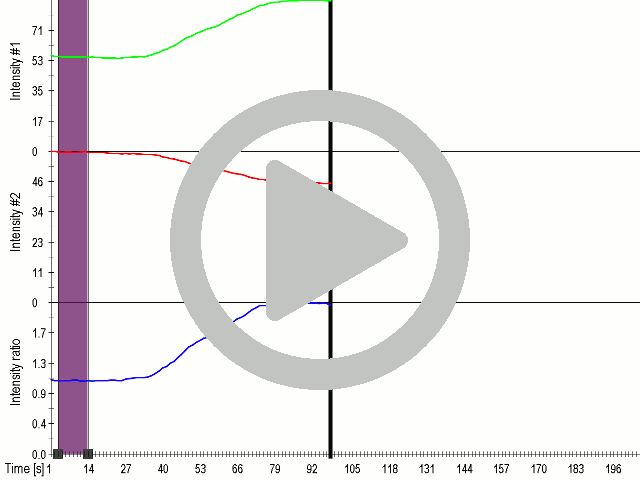
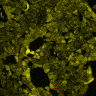


(A) Schematic presentation of a stimulation sequence and (B) signal parameters used for stimulation. (C) and (D) Cells expressing P3:FAS:TRPC1 showed increased sensitivity to ultrasound stimulation in comparison to the cells without exogenous mechanosensitive channel. HEK293 cells expressing P3:FAS:TRPC1 were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. For comparison cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
In order to observe mechanostimulation of cells with ectopically expressed mechanoreceptors we had to use high-power ultrasound. However, we tested that the cells nevertheless did not lose the viability by ultrasound stimulation. Our next challenge was to further improve sensitivity of cells to respond to lower power ultrasound as this would avoid stimulation of any endogenous channels and limit stimulation only to the engineered.



