| (21 intermediate revisions by 3 users not shown) | |||
| Line 151: | Line 151: | ||
<li><a href="/Team:NAWI-Graz/Human_Practices">Human Practices</a></li> | <li><a href="/Team:NAWI-Graz/Human_Practices">Human Practices</a></li> | ||
<li><a href="/Team:NAWI-Graz/Silver">Silver</a></li> | <li><a href="/Team:NAWI-Graz/Silver">Silver</a></li> | ||
| − | + | ||
| − | + | ||
</ul> | </ul> | ||
</li> | </li> | ||
<li class="dropdown-full-color dropdown-secondary"> | <li class="dropdown-full-color dropdown-secondary"> | ||
| − | <a href=" | + | <a href="/Team:NAWI-Graz/Sponsor"> |
Sponsoring | Sponsoring | ||
</a> | </a> | ||
| Line 175: | Line 174: | ||
<section class="section-custom-medical"> | <section class="section-custom-medical"> | ||
| − | + | ||
| − | + | ||
| − | <div class=" | + | |
| − | + | <div class="row"> | |
| − | < | + | <div class="col-md-12"> |
| − | + | <h4 class="mb-lg">Navigation</h4> | |
| − | + | ||
| + | |||
| + | |||
| + | <div class="row"> | ||
| + | <div class="col-md-4"> | ||
| + | <aside class="sidebar" id="sidebar" data-plugin-sticky data-plugin-options='{"minWidth": 991, "containerSelector": ".container", "padding": {"top": 110}}'> | ||
| + | |||
| + | <div class="tabs tabs-vertical tabs-left tabs-navigation"> | ||
| + | <ul class="nav nav-tabs col-sm-3"> | ||
| + | <li class="active"> | ||
| + | <a href="#tabsNavigation1" data-toggle="tab"><i class="fa fa-user"></i>Week 0</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation2" data-toggle="tab"><i class="fa fa-user"></i> Week 1</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation3" data-toggle="tab"><i class="fa fa-user"></i> Week 2</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation4" data-toggle="tab"><i class="fa fa-user"></i> Week 3</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation5" data-toggle="tab"><i class="fa fa-user"></i> Week 4</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation6" data-toggle="tab"><i class="fa fa-user"></i> Week 5</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation7" data-toggle="tab"><i class="fa fa-user"></i> Week 6</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation8" data-toggle="tab"><i class="fa fa-user"></i> Week 7</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation9" data-toggle="tab"><i class="fa fa-user"></i> Week 8</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation10" data-toggle="tab"><i class="fa fa-user"></i> Week 9</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation11" data-toggle="tab"><i class="fa fa-user"></i> Week 10</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation12" data-toggle="tab"><i class="fa fa-user"></i> Week 11</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation13" data-toggle="tab"><i class="fa fa-user"></i> Week 12</a> | ||
| + | </li> | ||
| + | <li> | ||
| + | <a href="#tabsNavigation14" data-toggle="tab"><i class="fa fa-user"></i> Week 13</a> | ||
| + | </li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | |||
| + | </aside> | ||
| + | </div> | ||
| + | <div class="col-md-8"> | ||
| + | <div class="tab-pane tab-pane-navigation active" id="tabsNavigation1"> | ||
| + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 0</span></h2> | ||
<p> | <p> | ||
Before the actual lab work was started the whole in silico had to be planed. | Before the actual lab work was started the whole in silico had to be planed. | ||
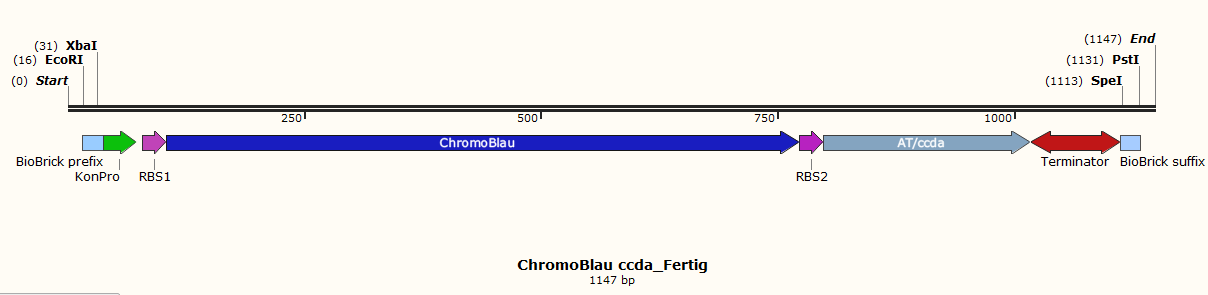
Therefore 10 different parts were designed. Each of them contains a biobrick suffix and a biobrick prefix, a promoter and ribosome binding sites. To this special bricks, unique restriction sites (XbaI, SpeI, EcoRI and PstI) are added for control digestion and directed ligation of the different parts. | Therefore 10 different parts were designed. Each of them contains a biobrick suffix and a biobrick prefix, a promoter and ribosome binding sites. To this special bricks, unique restriction sites (XbaI, SpeI, EcoRI and PstI) are added for control digestion and directed ligation of the different parts. | ||
| − | The toxin parts contain the tac-promotor because the toxin and the DAM methylase should be expressed after induction with IPTG. | + | The toxin parts (Fig.1, Fig.3, Fig.5 and Fig.7) contain the tac-promotor because the toxin and the DAM methylase should be expressed after induction with IPTG. |
With the Dam methylase a 50% higher mutation rate is obtained. | With the Dam methylase a 50% higher mutation rate is obtained. | ||
| − | The chromoprotein part possesses a constitutive promoter for the reason that the chromoprotein and the antitoxin should be expressed the whole time.</p> | + | The chromoprotein part (Fig.2, Fig.4, Fig.6 and Fig.8) possesses a constitutive promoter for the reason that the chromoprotein and the antitoxin should be expressed the whole time. |
| + | Other parts are the vector (Fig.10) and the lacI part (Fig.9).</p> | ||
| − | |||
| − | |||
| − | <img src="https://static.igem.org/mediawiki/2016/5/5a/T--NAWI-Graz--fig2.png" | + | <div class="image-gallery-item"> |
| − | < | + | <a href="https://static.igem.org/mediawiki/2016/3/3e/T--NAWI-Graz--Fig1.png" class="lightbox-portfolio"> |
| + | <span class="thumb-info"> | ||
| + | <span class="thumb-info-wrapper"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/3/3e/T--NAWI-Graz--Fig1.png" class="img-responsive" alt=""> | ||
| + | <span class="thumb-info-action"> | ||
| + | <span class="thumb-info-action-icon"><i class="fa fa-link"></i></span> | ||
| + | </span> | ||
| + | </span> | ||
| + | </span> | ||
| + | </a> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | <p>Fig. 1: ccdB toxin and the DAM methylase</p> | ||
| + | |||
| + | |||
| + | <div class="image-gallery-item"> | ||
| + | <a href="https://static.igem.org/mediawiki/2016/5/5a/T--NAWI-Graz--fig2.png" class="lightbox-portfolio"> | ||
| + | <span class="thumb-info"> | ||
| + | <span class="thumb-info-wrapper"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/5/5a/T--NAWI-Graz--fig2.png" class="img-responsive" alt=""> | ||
| + | <span class="thumb-info-action"> | ||
| + | <span class="thumb-info-action-icon"><i class="fa fa-link"></i></span> | ||
| + | </span> | ||
| + | </span> | ||
| + | </span> | ||
| + | </a> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | <p>Fig. 2: chromoprotein blue and the antitoxin ccdA</p> | ||
<img src="https://static.igem.org/mediawiki/2016/4/4e/T--NAWI-Graz--fig3.png" alt="fig3" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/4/4e/T--NAWI-Graz--fig3.png" alt="fig3" class="img-responsive"> | ||
| − | < | + | <p>Fig. 3 mazF toxin and DAM methylase</p> |
<img src="https://static.igem.org/mediawiki/2016/3/37/T--NAWI-Graz--fig4.png" alt="fig4" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/3/37/T--NAWI-Graz--fig4.png" alt="fig4" class="img-responsive"> | ||
| − | < | + | <p>Fig. 4 chromoprotein red and the antitoxin MazE</p> |
<img src="https://static.igem.org/mediawiki/2016/1/19/T--NAWI-Graz--fig5.png" alt="fig5" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/1/19/T--NAWI-Graz--fig5.png" alt="fig5" class="img-responsive"> | ||
| − | < | + | <p>Fig. 5: yafO toxin and DAM methylase</p> |
<img src="https://static.igem.org/mediawiki/2016/0/0e/T--NAWI-Graz--fig6.png" alt="fig6" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/0/0e/T--NAWI-Graz--fig6.png" alt="fig6" class="img-responsive"> | ||
| − | < | + | <p>Fig. 6: chromoprotein yellow and the antitoxin yafN</p> |
<img src="https://static.igem.org/mediawiki/2016/4/42/T--NAWI-Graz--fig7.png" alt="fig7" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/4/42/T--NAWI-Graz--fig7.png" alt="fig7" class="img-responsive"> | ||
| − | < | + | <p>Fig. 7: kid toxin and DAM methylase</p> |
<img src="https://static.igem.org/mediawiki/2016/e/ec/T--NAWI-Graz--fig8.png" alt="fig8" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/e/ec/T--NAWI-Graz--fig8.png" alt="fig8" class="img-responsive"> | ||
| − | < | + | <p>Fig. 8: chromoprotein green and the antitoxin kis</p> |
<img src="https://static.igem.org/mediawiki/2016/b/bf/T--NAWI-Graz--fig9.png" alt="fig9" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/b/bf/T--NAWI-Graz--fig9.png" alt="fig9" class="img-responsive"> | ||
| − | < | + | <p>Fig. 9: lacI gene</p> |
<img src="https://static.igem.org/mediawiki/2016/4/46/T--NAWI-Graz--fig10.png" alt="fig10" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/4/46/T--NAWI-Graz--fig10.png" alt="fig10" class="img-responsive"> | ||
| − | < | + | <p>Fig. 10: the vector, which consists an ampicillin resistence gene for selection</p> |
| Line 225: | Line 313: | ||
<p>For the following ligation the different parts are cut with different restriction enzymes. | <p>For the following ligation the different parts are cut with different restriction enzymes. | ||
| − | The first step is to cut the chromoprotein-parts with EcoRI and SpeI, the toxin parts with PstI and XbaI, and the vector with EcoRI and PstI.</p> | + | The first step is to cut the chromoprotein-parts with EcoRI and SpeI, the toxin parts with PstI and XbaI, and the vector with EcoRI and PstI (Fig.11).</p> |
<img src="https://static.igem.org/mediawiki/2016/6/68/T--NAWI-Graz--fig11.png" alt="fig11" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/6/68/T--NAWI-Graz--fig11.png" alt="fig11" class="img-responsive"> | ||
| − | < | + | <p>Fig. 11: The chromoprotein/antitoxin part and the toxin/DAM-methylase part are ligated into the vector</p> |
<p>The second step is that the lacI-gene is cloned into this construct for selectable reasons. | <p>The second step is that the lacI-gene is cloned into this construct for selectable reasons. | ||
| − | Therefore the cloned vector and the lacI-gene are cut with PstI.</p> | + | Therefore the cloned vector and the lacI-gene are cut with PstI (Fig.12).</p> |
<img src="https://static.igem.org/mediawiki/2016/4/4b/T--NAWI-Graz--fig12.png" alt="fig12" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/4/4b/T--NAWI-Graz--fig12.png" alt="fig12" class="img-responsive"> | ||
| − | < | + | <p>Fig. 12: The finished vector</p> |
| + | |||
| + | |||
| + | </div> | ||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation2"> | ||
| + | |||
<h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 1 (11.07 – 15.07)</span></h2> | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 1 (11.07 – 15.07)</span></h2> | ||
| Line 248: | Line 341: | ||
| − | + | </div> | |
| − | + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation3"> | |
| + | |||
| + | |||
<h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 2 (18.07 – 22.07)</span></h2> | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 2 (18.07 – 22.07)</span></h2> | ||
<p><b>Test-Transformation & pJET cloning of gBlocks</p></b> | <p><b>Test-Transformation & pJET cloning of gBlocks</p></b> | ||
| Line 259: | Line 354: | ||
| − | + | </div> | |
| − | + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation4"> | |
| + | |||
| + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 3 (25.07 – 29.07)</span></h2> | ||
| + | <p><b>Preparing gBlocks for sequencing</b></p> | ||
| − | <p>By using the optimized blunt-end cloning protocol we finally received first positive transformation results, more specifically colonies. After doing dilution plating (5 of each strand) we also prepared ONCs out of the colonies for plasmid isolation. </p> | + | <p>By using the optimized blunt-end cloning protocol we finally received first positive transformation results, more specifically colonies. After doing dilution plating (5 of each strand) (Fig. 13 and Fig. 14) we also prepared ONCs out of the colonies for plasmid isolation. </p> |
| − | <p>Another task of the week was to prepare glucose medium, by adding glucose to our LB medium, for repression of the promotors to keep the toxin down. Otherwise they would kill themselves already in the first step of cloning. </p> | + | <p>Another task of the week was to prepare glucose medium, by adding glucose to our LB medium, for repression of the promotors to keep the toxin down. Otherwise they would kill themselves already in the first step of cloning. </p> |
| − | <p>Furthermore, at the end of the week, new diluting plating was done because the first ones did not grow in a proper way. </p> | + | <p>Furthermore, at the end of the week, new diluting plating was done because the first ones did not grow in a proper way. </p> |
| − | <img src="https://static.igem.org/mediawiki/2016/2/2d/T--NAWI-Graz--fig13.png" alt="fig13" class="img-responsive"> | + | <img src="https://static.igem.org/mediawiki/2016/2/2d/T--NAWI-Graz--fig13.png" alt="fig13" class="img-responsive"> |
| − | < | + | <p>Fig 13: pJET Blue diluting plate (before sequencing)</p> |
| − | <img src="https://static.igem.org/mediawiki/2016/c/ce/T--NAWI-Graz--fig14.png" alt="fig14" class="img-responsive"> | + | <img src="https://static.igem.org/mediawiki/2016/c/ce/T--NAWI-Graz--fig14.png" alt="fig14" class="img-responsive"> |
| − | < | + | <p>Fig 14: on the left side you can see the blue and the red diluting plates, |
| − | which also were working, not all of the other diluting plating were working well, just mazE.</ | + | which also were working, not all of the other diluting plating were working well, just mazE.</p> |
| + | </div> | ||
| + | |||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation5"> | ||
| + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 4 (1.08 - 5.08)</span></h2> | ||
| + | <p><b>Sequencing results and PCR</b></p> | ||
| + | <p>New ONCs of all 10 cloned gBlocks (in pJETs) were done from our diluting plating. To isolate the plasmid–DNA we used the GeneJET Plasmid Miniprep Kit. To get the concentration of the extracted DNA NanoDrop was used and showed results from 22 ng/µl up to 224 ng/µl. </p> | ||
| − | + | <p>We sent 60 – 80 ng of each gBlock–pJET DNA with the proper primers for sequencing to Microsynth Austria GmbH. The following day, we analyzed the sequencing results and saw that the gBlock red, blue, lacI and mazF had the correct DNA–sequence (Fig. 15). The other gBlocks showed no matches (Fig. 16). Because of the fact that the other gBlocks didn`t show the expected results, we changed our plan and started to use pUC19 (Fig. 17) instead of our own designed vector and our lacI part. Unfortunately, we didn’t have enough gBlocks left for further experiments. So we decided to run a PCR, followed by a gel electrophoresis.</p> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | <p> | + | <p>The gel didn’t show clear bands at the appropriate size. </p> |
| − | <p> | + | <img src="https://static.igem.org/mediawiki/2016/f/fb/T--NAWI-Graz--fig15.png" alt="fig15" class="img-responsive"> |
| + | <p>Fig 15: The sequencing results of the red part. the third clone showed the best results, so subsequent works were done with clone 3.</p> | ||
| − | <p> | + | <img src="https://static.igem.org/mediawiki/2016/1/1a/T--NAWI-Graz--fig16.png" alt="fig16" class="img-responsive"> |
| + | <p>Fig 16: This is an example how bad our sequencing results were. In the case of the vector we could work together.</p> | ||
| − | < | + | |
| − | < | + | </div> |
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation6"> | ||
| + | |||
| + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 5 (08.08 – 12.08)</span></h2> | ||
| + | <p><b>PCR of gBlocks for directed cloning into pUC19</b></p> | ||
| − | < | + | <p>To see the quality of our delivered gBlock–DNA, we ran a gel electrophoresis using pure gBlock-DNA. The surprising results have shown that there were some samples with very less DNA and even some with no DNA at the appropriate size. The samples that have shown a band were cut out at the correct length and resolved, followed by a PCR reaction: </p> |
| − | + | ||
| + | <p>98 °C 30 sec<br> | ||
| + | 98 °C 10 sec I<br> | ||
| + | 68 °C 20 sec I 30 x<br> | ||
| + | 72 °C 30 sec I<br> | ||
| + | 72 °C 7 min<br> | ||
| + | 4 °C ∞</p> | ||
| − | + | <p>Because of the fact that some gBlocks didn’t show results at all, we asked IDT for new samples and they sent ccdB toxin & our designed vector again. </p> | |
| − | + | ||
| − | + | ||
| − | <p> | + | <p>Moreover, a new attempt of cloning ccdB into a pJET vector was performed with the last few µl of the original sample. After transformation colonies were detected and used for a raster-screening, to double-check the size of our gBlock DNA. The correct ones were used for a colony PCR as following: </p> |
| − | <p>98 °C | + | <p>98 °C 5 min<br> |
| − | 98 °C 10 sec I<br> | + | 98 °C 10 sec I<br> |
| − | 68 °C 20 sec I | + | 68 °C 20 sec I 36 x<br> |
| − | 72 °C 30 sec I<br> | + | 72 °C 30 sec I<br> |
| − | 72 °C 7 min<br> | + | 72 °C 7 min<br> |
| − | 4 °C ∞</p> | + | 4 °C ∞</p> |
| − | + | <img src="https://static.igem.org/mediawiki/2016/d/d5/T--NAWI-Graz--fig17.png" alt="fig17" class="img-responsive"> | |
| − | + | <p>Fig 17: The pUC19 vector we choose to work together, it has the restriction sites and the characteristics we need.</p> | |
| − | + | </div> | |
| − | + | ||
| − | + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation7"> | |
| − | + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 6 (16.08 – 19.08)</span></h2> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | < | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<p><b>Miniprep & Sequencing of pUC19</b></p> | <p><b>Miniprep & Sequencing of pUC19</b></p> | ||
| − | <p>As described above, the pUC19 vector was used and provided on plates, by our department. To get the DNA of pUC19 miniprep was done. Also, a miniprep of the apparently correct ccdB from the raster-screening was done. After that, the DNA was sent to sequencing and the results have shown that the toxin (ccdB) was not in the sequence anymore. It was probably looped out by the cells because of its toxicity as a result of the leaky tac-promotor. It was possible because two identical ribosome binding sites at the beginning and at the end of the sequence were designed. </p> | + | <p>As described above, the pUC19 vector was used and provided on plates, by our department. To get the DNA of pUC19 miniprep was done. Also, a miniprep of the apparently correct ccdB from the raster-screening was done. After that, the DNA was sent to sequencing and the results have shown that the toxin (ccdB) was not in the sequence anymore (Fig. 18). It was probably looped out by the cells because of its toxicity as a result of the leaky tac-promotor. It was possible because two identical ribosome binding sites at the beginning and at the end of the sequence were designed. </p> |
<img src="https://static.igem.org/mediawiki/2016/b/be/T--NAWI-Graz--fig18.png" alt="fig18" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/b/be/T--NAWI-Graz--fig18.png" alt="fig18" class="img-responsive"> | ||
| − | < | + | <p>Fig 18: In this figure is shown that the toxin was looped out.</p> |
| Line 345: | Line 441: | ||
| − | + | </div> | |
| − | + | ||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation8"> | ||
| + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 7 (22.08 – 26.08)</span></h2> | ||
<p><b>Restriction & Ligation of the parts</b></p> | <p><b>Restriction & Ligation of the parts</b></p> | ||
| Line 361: | Line 459: | ||
<p>For this reason, new restriction and ligation attempts were performed. To get a greater chance of correct colonies 3-point-ligation was done additionally. Another try was to use less PCR product. Still no result was as expected. </p> | <p>For this reason, new restriction and ligation attempts were performed. To get a greater chance of correct colonies 3-point-ligation was done additionally. Another try was to use less PCR product. Still no result was as expected. </p> | ||
| − | + | ||
| − | + | </div> | |
| + | |||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation9"> | ||
| + | |||
<h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 8 (29.08 – 02.09)</span></h2> | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 8 (29.08 – 02.09)</span></h2> | ||
<p><b>Restriction & Ligation, new ccdB & BioBricks</b></p> | <p><b>Restriction & Ligation, new ccdB & BioBricks</b></p> | ||
| − | <p>Over the weekend the diluting plating plates from the week before surprisingly got red. For this reason, we performed a colony PCR, followed by gel electrophoresis, to check the size of the bands. A ONC was also done to do a miniprep. After measuring the amount of DNA the plasmids were cut by PstI and Xbal. To check if both inserts and the vector were present in the plasmid a gel electrophoresis was done. Only one sample showed the expected results. </p> | + | <p>Over the weekend the diluting plating plates from the week before surprisingly got red. For this reason, we performed a colony PCR, followed by gel electrophoresis, to check the size of the bands. A ONC was also done to do a miniprep and a aliquote was plated (Fig. 19). After measuring the amount of DNA the plasmids were cut by PstI and Xbal. To check if both inserts and the vector were present in the plasmid a gel electrophoresis was done. Only one sample showed the expected results. </p> |
<img src="https://static.igem.org/mediawiki/2016/f/f7/T--NAWI-Graz--fig19.png" alt="fig19" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/f/f7/T--NAWI-Graz--fig19.png" alt="fig19" class="img-responsive"> | ||
| − | < | + | <p>Fig 19: Red colonies</p> |
| Line 378: | Line 479: | ||
<p>Furthermore, our team started to particularly working on the BioBricks. It started with pJET-cloning and -transformation. </p> | <p>Furthermore, our team started to particularly working on the BioBricks. It started with pJET-cloning and -transformation. </p> | ||
| − | + | </div> | |
| − | + | ||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation10"> | ||
| + | |||
<h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 9 (05.09 – 09.09)</span></h2> | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 9 (05.09 – 09.09)</span></h2> | ||
<p><b>Restriction & Ligation, pJET & Sequencing of new ccdB</b></p> | <p><b>Restriction & Ligation, pJET & Sequencing of new ccdB</b></p> | ||
| Line 390: | Line 493: | ||
| − | + | </div> | |
| − | + | ||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation11"> | ||
| + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 10 (12.09 – 16.09)</span></h2> | ||
<p><b>Miniprep & Protein Expression Control</b></p> | <p><b>Miniprep & Protein Expression Control</b></p> | ||
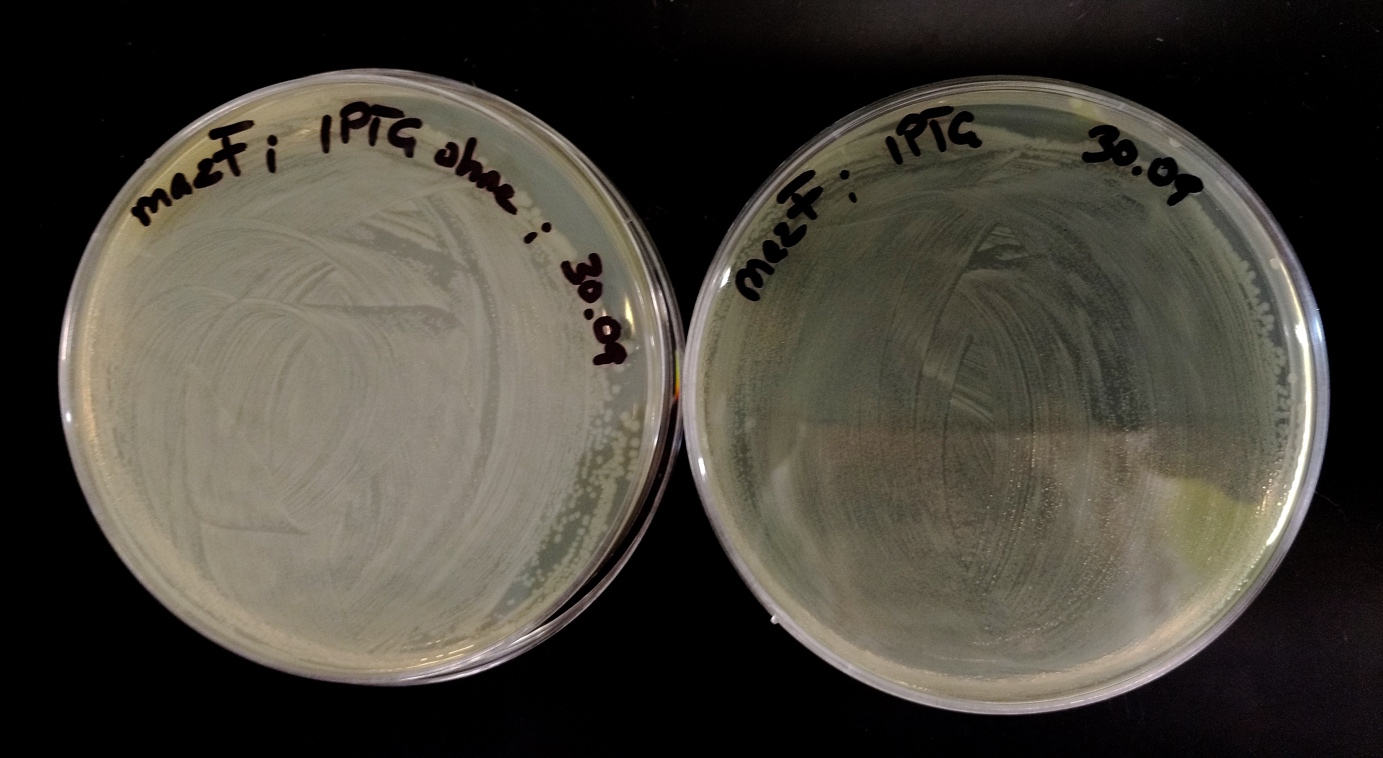
| − | <p>Minipreps of week 9 were digested with PstI and XbaI but rejected for the following reason: Blue colonies were all of a sudden spotted on the plates of the transformation of week 9. For this reason, new diluting plating was done with 8 blue colonies. ONCs were made by inoculating the same colonies. The next day miniprep was performed. After measuring nanodrop a restriction digestion for controlling the presence of all three inserts was done. The last three of the 8 bands showed the correct size and were sent to sequencing. Band number 7 was correct. </p> | + | <p>Minipreps of week 9 were digested with PstI and XbaI but rejected for the following reason: Blue colonies were all of a sudden spotted on the plates of the transformation of week 9 (Fig. 20). For this reason, new diluting plating was done with 8 blue colonies (Fig. 21). ONCs were made by inoculating the same colonies. The next day miniprep was performed. After measuring nanodrop a restriction digestion for controlling the presence of all three inserts was done (Fig. 22). The last three of the 8 bands showed the correct size and were sent to sequencing. Band number 7 was correct (Fig. 23. </p> |
<img src="https://static.igem.org/mediawiki/2016/2/2c/T--NAWI-Graz--fig20.png" alt="fig20" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/2/2c/T--NAWI-Graz--fig20.png" alt="fig20" class="img-responsive"> | ||
| − | < | + | <p>Fig 20: Some blue colonies were detected</p> |
<img src="https://static.igem.org/mediawiki/2016/1/1a/T--NAWI-Graz--fig21.png" alt="fig21" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/1/1a/T--NAWI-Graz--fig21.png" alt="fig21" class="img-responsive"> | ||
| − | < | + | <p>Fig 21: The correct blue one</p> |
<img src="https://static.igem.org/mediawiki/2016/c/c0/T--NAWI-Graz--fig22.png" alt="fig22" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/c/c0/T--NAWI-Graz--fig22.png" alt="fig22" class="img-responsive"> | ||
| − | < | + | <p>Fig 22: <i>E.Coli</i> XL1/pUC19_blue_ccdB</p> |
<img src="https://static.igem.org/mediawiki/2016/0/0b/T--NAWI-Graz--fig23.png" alt="fig23" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/0/0b/T--NAWI-Graz--fig23.png" alt="fig23" class="img-responsive"> | ||
| − | < | + | <p>Fig 23: Sequencing results of the correct blue one</p> |
| Line 415: | Line 520: | ||
| − | + | </div> | |
| − | + | ||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation12"> | ||
| + | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 11 (19.09 – 23.09)</span></h2> | ||
<p><b>Red_mazF & Protein Expression Control </b></p> | <p><b>Red_mazF & Protein Expression Control </b></p> | ||
| − | <p>SDS-Gel was run with MOPS at 200 V for 50 min. Afterwards, the gel was colored by coloring solution. After approximately one hour bands could be seen on the gel, but no bands for proteins under 10 kDa. To see also these proteins, we did the SDS-Gel again, shortened the runtime of the SDS and used MES-buffer instead of MOPS-buffer.</p> | + | <p>SDS-Gel was run with MOPS at 200 V for 50 min. Afterwards, the gel was colored by coloring solution. After approximately one hour bands could be seen on the gel, but no bands for proteins under 10 kDa. To see also these proteins, we did the SDS-Gel again, shortened the runtime of the SDS and used MES-buffer instead of MOPS-buffer (Fig. 24).</p> |
<img src="https://static.igem.org/mediawiki/2016/5/56/T--NAWI-Graz--fig24.png" alt="fig24" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/5/56/T--NAWI-Graz--fig24.png" alt="fig24" class="img-responsive"> | ||
| − | < | + | <p>Figure 24: SDS-Gel of proteins that were isolated with sonication followed by centrifugation. Standard: PageRuler Prestained Protein Ladder. Protein size of chromoprotein blue: 24,5 kDa; Chromoprotein red: 25,6 kDa; Toxin ccdB: 11,7 kDa, Antitoxin ccdA: 8,3 kDa; Toxin mazF: 7,7 kDa; Antitoxin mazE: 9,4 kDa; DAM methylase: 32 kDa.</p> |
| Line 430: | Line 537: | ||
| − | <p>Because of the fact, that we got in the tenth week Red_mazF with the right insert in our transformants, we decided to control the transformants of this week again. So we looked up for the right plates, picked eight red colonies, streaked them out, made an ONC and cut it with EcoRI. Then we performed a agrose-gel electrophoresis and surprisingly some of these colonies showed the right band size. </p> | + | <p>Because of the fact, that we got in the tenth week Red_mazF with the right insert in our transformants, we decided to control the transformants of this week again. So we looked up for the right plates, picked eight red colonies, streaked them out, made an ONC and cut it with EcoRI. Then we performed a agrose-gel electrophoresis and surprisingly some of these colonies showed the right band size (Fig. 25). </p> |
<img src="https://static.igem.org/mediawiki/2016/0/06/T--NAWI-Graz--fig25.png" alt="fig25" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/0/06/T--NAWI-Graz--fig25.png" alt="fig25" class="img-responsive"> | ||
| − | < | + | <p>Figure 25: Agarose Gel of eight different Red_mazF colonies from plate X, cut with EcorI. Standard: GeneRulerTM 1 kb DNA Ladder.</p> |
| Line 440: | Line 547: | ||
| − | <p>Six out of eight colonies showed the right bands (~4000 bp and ~1000 bp). Clone 6 and clone 7 only show one band at ~4000 bp. Seems like there was no insert in these clones. </p> | + | <p>Six out of eight colonies showed the right bands (~4000 bp and ~1000 bp). Clone 6 and clone 7 only show one band at ~4000 bp. Seems like there was no insert in these clones. The correct red one was streaked out again (Fig. 26). </p> |
<img src="https://static.igem.org/mediawiki/2016/d/d0/T--NAWI-Graz--fig26.png" alt="fig26" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/d/d0/T--NAWI-Graz--fig26.png" alt="fig26" class="img-responsive"> | ||
| − | < | + | <p>Fig 26: The correct red one</p> |
| − | <p>Comparing the results from the gelelectrophoresis with the streaked colonies (fig. | + | <p>Comparing the results from the gelelectrophoresis with the streaked colonies (fig. 27), one can see that mostly light-red colonies showed the right plasmid-size. Maybe this was the reason why we didn´t find Red_mazF with the proper plasmid in the tenth week anymore, because we always analyzed colonies that grew dark-red. </p> |
<img src="https://static.igem.org/mediawiki/2016/5/53/T--NAWI-Graz--fig27.png" alt="fig27" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/5/53/T--NAWI-Graz--fig27.png" alt="fig27" class="img-responsive"> | ||
| − | < | + | <p>Fig. 27: Eight re-streaked colonies (LB-amp plates) from a plate with Red_mazF transformants from week 10.</p> |
| − | + | </div> | |
| + | |||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation13"> | ||
| + | |||
<h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 12 (26.09 – 30.09)</span></h2> | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 12 (26.09 – 30.09)</span></h2> | ||
<p><b>Protein expression control in culture media</b></p> | <p><b>Protein expression control in culture media</b></p> | ||
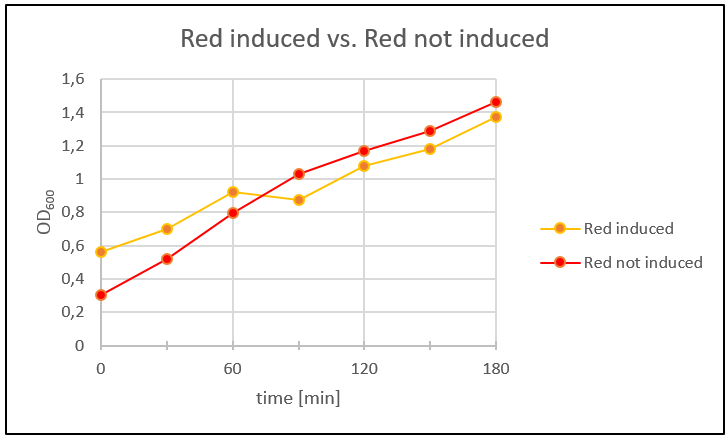
| − | <p>Because the SDS-Gel of the proteins wasn´t that clear, so we couldn´t say if the toxins were produced or not. We analyzed the protein expression in flasks with LB-media. Therefore we prepared an ONC of Blue_ccdB and Red_mazF and let the ONC's grow to OD600 ~0,1. Four main cultures were made: two with Blue_ccdB and two with Red_mazF. We let them grow to OD600 ~0,5. When the OD600 was reached we induced one main culture from Blue_ccdB and one from Red_mazF with IPTG (1 mM). We took a sample of our main cultures every 30 minutes over several hours and measured the OD600. Additionally we plated 100 µl of each culture on a LB-Agar plate (dilution 1:100; Amp 50µg/ml). The plates were incubated over night at 37°C. </p> | + | <p>Because the SDS-Gel of the proteins wasn´t that clear, so we couldn´t say if the toxins were produced or not. We analyzed the protein expression in flasks with LB-media. Therefore we prepared an ONC of Blue_ccdB and Red_mazF and let the ONC's grow to OD600 ~0,1. Four main cultures were made: two with Blue_ccdB and two with Red_mazF. We let them grow to OD600 ~0,5. When the OD600 was reached we induced one main culture from Blue_ccdB and one from Red_mazF with IPTG (1 mM). We took a sample of our main cultures every 30 minutes over several hours and measured the OD600 (Fig. 28 and Fig. 29). Additionally we plated 100 µl of each culture on a LB-Agar plate (dilution 1:100; Amp 50µg/ml). The plates were incubated over night at 37°C. </p> |
<img src="https://static.igem.org/mediawiki/2016/b/b2/T--NAWI-Graz--fig28.png" alt="fig28" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/b/b2/T--NAWI-Graz--fig28.png" alt="fig28" class="img-responsive"> | ||
| − | < | + | <p>Figure 28: One Red_mazF main culture was induced with IPTG (1 mM).To compaire the results the other main culture wasn’t induced with IPTG. The OD600 was measured every 30 minutes over 3 hours. </p> |
<img src="https://static.igem.org/mediawiki/2016/9/9c/T--NAWI-Graz--fig29.png" alt="fig29" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/9/9c/T--NAWI-Graz--fig29.png" alt="fig29" class="img-responsive"> | ||
| − | < | + | <p>Figure 29: One Blue_ccdB main culture was induced with IPTG (1 mM). To compare the results the other main culture wasn’t induced with IPTG. The OD600 was measured every 30 minutes over 2.5 hours.</p> |
| − | <p>In figure | + | <p>In figure 28 and figure 29 the time was plotted against the measured OD600 to get a growth curve of the cells. The Blue_ccdb induced curve is lower than the not induced Blue_ccdB curve. Therefore we can say that the toxin in Blue_ccdB induced was produced. Additionally we can see that our cells in the not induced culture grew better than the induced culture. Red_mazF didn´t show the same result. Red_mazF induced was growing better than Red_mazF not induced. After 60 minutes Red_mazF induced grew slower than Red_mazF not induced but figure 4 doesn’t show the expected result. Maybe that’s because our cells didn’t express the toxin. This could be mostly the reason, because we used a red colony for this experiment that came from the plate with re-streaked transformants from week 10. The colonies on this plate only showed the right plasmid-size in the beginning but not later on. |
| − | Next day we counted the colonies on the LB-amp plates and got following results:</p> | + | Next day we counted the colonies on the LB-amp plates and got following results (table 1):</p> |
| − | < | + | <p>Table 1: Number of colonies. Over 3 hours samples were taken every 30 minutes from our main cultures Blue_ccdB induced, Blue_ccdB not induced, Red_mazF induced and Red_mazF not induced. 100 µl of the cultures were plated on LB-Agar plates (50 µg/ml Amp; 1:100 dilution).</p> |
| Line 535: | Line 645: | ||
<p>Looking at the numbers from table 1, we can see that Blue_ccdB and Red_mazF not induced got a lot more colonies than Blue_ccdB and Red_mazF induced. Hence the induced cultures were able to produce the protein and were growing slower because of that, while the not induced cultures continued growing. But the results of our red cultures were not comparable with the results we got in figure 4. | <p>Looking at the numbers from table 1, we can see that Blue_ccdB and Red_mazF not induced got a lot more colonies than Blue_ccdB and Red_mazF induced. Hence the induced cultures were able to produce the protein and were growing slower because of that, while the not induced cultures continued growing. But the results of our red cultures were not comparable with the results we got in figure 4. | ||
We decided to repeat the same experiment with our red cultures. </p> | We decided to repeat the same experiment with our red cultures. </p> | ||
| − | <p>We started a new protein expression experiment in culture media, with Red_mazF | + | <p>We started a new protein expression experiment in culture media, with Red_mazF that showed the right plasmid-size on the gel. We prepared ONC´s and the next day we made two main cultures out of the ONC. In this experiment the main culture was already at the beginning induced with IPTG (1mM). Over several hours the OD600 was measured.</p> |
<img src="https://static.igem.org/mediawiki/2016/2/2e/T--NAWI-Graz--fig30.png" alt="fig30" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/2/2e/T--NAWI-Graz--fig30.png" alt="fig30" class="img-responsive"> | ||
| − | < | + | <p>Figure 30: One Red_mazF main culture was induced with IPTG (1mM) the other one not. Over 3.5 hourss the OD600 was measured.</p> |
| − | <p>Figure | + | <p>Figure 30 shows that the not induced culture (orange line) grew better than the induced culture (red line). Therefore it's possible to say that the induced culture expressed the toxin and so the cells were inhibited in growth. |
At the beginning both cultures had nearly the same growth. After 2.5 hours it´s possible to see the difference between the induced and not induced culture. | At the beginning both cultures had nearly the same growth. After 2.5 hours it´s possible to see the difference between the induced and not induced culture. | ||
Figure 6 shows that after a while it's clear that the induced culture didn´t grow in the same way like the not induced culture. We can say that was because of the toxin expression in the induced culture. It inhibited the growth of the cells. Hence we have cloned the right gene and also the right protein was expressed compared to our previous results. </p> | Figure 6 shows that after a while it's clear that the induced culture didn´t grow in the same way like the not induced culture. We can say that was because of the toxin expression in the induced culture. It inhibited the growth of the cells. Hence we have cloned the right gene and also the right protein was expressed compared to our previous results. </p> | ||
| − | + | </div> | |
| + | |||
| + | <div class="tab-pane tab-pane-navigation" id="tabsNavigation14"> | ||
| + | |||
<h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 13 (03.10 – 07.10)</span></h2> | <h2 class="mt-xl mb-none"><span class="alternative-font font-size-md">Week 13 (03.10 – 07.10)</span></h2> | ||
<p><b>Red_mazF vs. Blue_ccdB on LB-amp plates</b></p> | <p><b>Red_mazF vs. Blue_ccdB on LB-amp plates</b></p> | ||
| Line 553: | Line 666: | ||
<img src="https://static.igem.org/mediawiki/2016/0/05/T--NAWI-Graz--fig31.png" alt="fig31" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/0/05/T--NAWI-Graz--fig31.png" alt="fig31" class="img-responsive"> | ||
| − | < | + | <p>Figure 31: LB-amp plate 1 and 2: Six (1,2,3,4,5 and 8) colonies with proven positiv insert |
(see fig. 2 and 3). LB-amp plate 3 (plated with 20 µl IPTG (1 M)): 1.1 Red_mazF and 1.1 Blue_ccdB | (see fig. 2 and 3). LB-amp plate 3 (plated with 20 µl IPTG (1 M)): 1.1 Red_mazF and 1.1 Blue_ccdB | ||
(also with proven positiv insert) were streaked one upon the other. LB-amp plate 4: 1.1 Red_mazF | (also with proven positiv insert) were streaked one upon the other. LB-amp plate 4: 1.1 Red_mazF | ||
and 1.1 Blue_ccdB. LB-amp plate 5: 2.4 Red_mazF and 1.1 Blue_ccdB. LB-amp plate 6: 1.1 Red_mazF, | and 1.1 Blue_ccdB. LB-amp plate 5: 2.4 Red_mazF and 1.1 Blue_ccdB. LB-amp plate 6: 1.1 Red_mazF, | ||
| − | 2.4 Red_mazF and 1.1 Blue_ccdB.</ | + | 2.4 Red_mazF and 1.1 Blue_ccdB.</p> |
| Line 570: | Line 683: | ||
than its fellow 2.4 Red_mazF could be the fact, that 1.1 Red_mazF didn´t produce as much chromoprotein | than its fellow 2.4 Red_mazF could be the fact, that 1.1 Red_mazF didn´t produce as much chromoprotein | ||
red than 2.4 Red_mazF. Because of that 1.1 Red_mazF had less to do and thus more time for other | red than 2.4 Red_mazF. Because of that 1.1 Red_mazF had less to do and thus more time for other | ||
| − | methabolic reactions, what made Red_mazF stronger. </p> | + | methabolic reactions, what made Red_mazF stronger (Fig. 31). </p> |
<img src="https://static.igem.org/mediawiki/2016/8/84/T--NAWI-Graz--fig32.png" alt="fig32" class="img-responsive"> | <img src="https://static.igem.org/mediawiki/2016/8/84/T--NAWI-Graz--fig32.png" alt="fig32" class="img-responsive"> | ||
| − | < | + | <p>Fig. 32: The not induced red one and the induced red one. </p> |
| − | + | </div> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</div> | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| − | + | ||
| + | |||
| + | |||
</section> | </section> | ||
<footer id="footer" class="m-none"> | <footer id="footer" class="m-none"> | ||
<div class="container"> | <div class="container"> | ||
| + | |||
| + | |||
| + | <div class="row mb-xlg"> | ||
| + | <div class="col-md-12"> | ||
| + | |||
| + | <div class="row show-grid"> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/b/b2/T--NAWI-Graz--StefitzLogo.png" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/9/9f/Thermo-Fisher.jpeg" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/1/1a/Zeta.jpeg" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/0/00/Microsynth.jpeg" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/3/3b/T--NAWI-Graz--Schursches-Insel-Cafe-Logo.jpeg" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/3/39/T--NAWI-Graz--snapgene.png" alt class="img-responsive" /> </span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/e/e5/KFU.jpeg" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/5/5f/TU_Graz.jpeg" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/7/76/T--NAWI-Graz--servolab.png" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/1/1e/T--NAWI-Graz--bartelt.png" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/4/44/T--NAWI-Graz--greiner-logo.png" alt class="img-responsive" /></span></div> | ||
| + | <div class="col-md-1"><span class="show-grid-block"><img src="/wiki/images/1/11/T--NAWI-Graz--IDT-Logo.png" alt class="img-responsive" /></span></div> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
<div class="row"> | <div class="row"> | ||
<div class="col-md-3"> | <div class="col-md-3"> | ||
| Line 632: | Line 733: | ||
8010 Graz, AUT<br> | 8010 Graz, AUT<br> | ||
</p> | </p> | ||
| + | </div> | ||
| + | <div class="col-md-3 col-md-offset-1"> | ||
| + | <div class="contact-details"> | ||
| + | <h4 class="mb-xlg">Mail address</h4> | ||
| + | <a class="text-decoration-none" href="mailto:igem.nawigraz@gmail.com"> | ||
| + | igem.nawigraz@gmail.com | ||
| + | </a> | ||
| + | </div> | ||
</div> | </div> | ||
<div class="col-md-2"> | <div class="col-md-2"> | ||
| Line 655: | Line 764: | ||
</div> | </div> | ||
</footer> | </footer> | ||
| + | |||
| + | |||
</div> | </div> | ||
<!-- Vendor --> | <!-- Vendor --> | ||
Latest revision as of 17:44, 19 October 2016