| (2 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
</html> {{Team:Tel-Hai/Header}} <html> | </html> {{Team:Tel-Hai/Header}} <html> | ||
| − | <h3> | + | <h3>Description</h3> |
| − | <div class=" | + | <div class="description"> |
| − | < | + | <div class="image-holder"><img src="/wiki/images/8/86/T--Tel-Hai--des1.png" /></div> |
| − | < | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | </div> | + | |
| − | < | + | <h3>Cystic fibrosis (CF)</h3> |
| + | <p>CF is a genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. Long-term issues include difficulty breathing and coughing up mucus as a result of frequent lung infections. Other signs and symptoms include sinus infections, poor growth, fatty stool, clubbing of the fingers and toes, and infertility in males, among others. </p> | ||
| + | <p>CF is caused by a mutation in the gene cystic fibrosis transmembrane conductance regulator (CFTR). The most common mutation, ΔF508, is a deletion (Δ signifying deletion) of three nucleotides that results in a loss of the amino acid phenylalanine (F) at the 508th position on the protein. This mutation accounts for two-thirds (66–70%) of CF cases worldwide and 90% of cases in the United States; however, over 1500 other mutations can produce CF.</p> | ||
| + | <div class="image-holder"><img src="/wiki/images/6/63/T--Tel-Hai--des2.png" /></div> | ||
| + | <p>There is no known cure for Cystic Fibrosis. Lung infections are treated with antibiotics which may be given intravenously, inhaled, or orally. People with the disease take between 50-80 medications per day, and on a daily basis partake in physical therapy in order to facilitate the lungs, inhalation treatments to relieve respiratory distress, and are hospitalized every couple of months.</p> | ||
| + | <p> Over the years, many drugs have been developed to help patients cope with symptoms and increase life expectancy. However, survival age is still only 40 years, as of 2015. In other words, these people are born knowing that they will not have a long life. </p> | ||
| + | <div class="image-holder"><img src="/wiki/images/7/79/T--Tel-Hai--des3.png" /></div> | ||
| + | <p><strong>Our group has focused on solving the root cause i.e. correcting the CFTR mutation. Thus, we were looking for gene editing technology.</strong></p> | ||
| − | <p> | + | <h3>CRISPR / Cas9</h3> |
| + | <p>The CRISPR/Cas system is a prokaryotic immune system that confers resistance to foreign genetic elements and provides a form of acquired immunity. CRISPR associated proteins (Cas) use the CRISPR spacers to recognize and cut these exogenous genetic elements in a manner analogous to RNA interference in eukaryotic organisms.</p> | ||
| + | <p> By delivering the Cas9 nuclease and appropriate guide RNAs into a cell, the cell's genome can be cut at a desired location, allowing existing genes to be removed and/or new ones to be added by homologous recombination resulting in the correction of the CFTR mutation.</p> | ||
| + | <div class="image-holder"><img src="/wiki/images/7/79/T--Tel-Hai--des4.png" /></div> | ||
| + | <p><strong>Next, our group though about a way to deliver CRISPR/Cas9 into the mutated cells.</strong></p> | ||
| − | <h3> | + | <h3>Cholera Toxin (CT)</h3> |
| − | <p> | + | <p>CT is composed of two molecular subunits: Cholera toxin subunit A (CTA) and B (CTB). CTA is responsible for the toxic effect, whereas the non-toxic subunit B is responsible for the internalization and transport of the toxin into the cell. </p> |
| − | + | <p> CTB is highly specific to epithelial cells (those are the mutated cells in CF). With its ability to bind the ganglioside receptor (GM1) upon the epithelial cell membrane, we believe it will become a successful carrier for introducing the plasmids of the CRISPR into the cells. | |
| − | < | + | .</p> |
| − | + | <div class="image-holder"><img src="/wiki/images/5/52/T--Tel-Hai--des5.png" /></div> | |
| − | </p> | + | <p><strong>In this project, we are combining these three elements together to develop a delivery system that can cure Cystic Fibrosis with a single treatment.</strong></p> |
| + | </div> | ||
</div></html> | </div></html> | ||
Latest revision as of 20:41, 19 October 2016

iGEM Tel-Hai 2016
Description
Cystic fibrosis (CF)
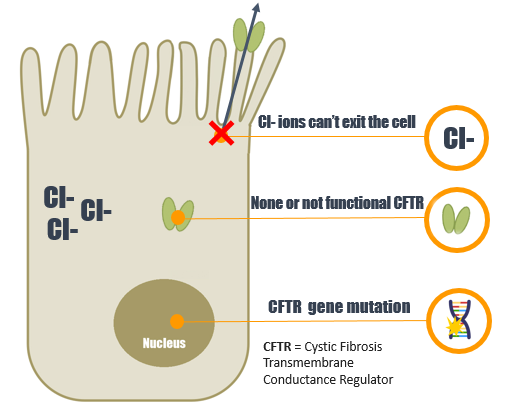
CF is a genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. Long-term issues include difficulty breathing and coughing up mucus as a result of frequent lung infections. Other signs and symptoms include sinus infections, poor growth, fatty stool, clubbing of the fingers and toes, and infertility in males, among others.
CF is caused by a mutation in the gene cystic fibrosis transmembrane conductance regulator (CFTR). The most common mutation, ΔF508, is a deletion (Δ signifying deletion) of three nucleotides that results in a loss of the amino acid phenylalanine (F) at the 508th position on the protein. This mutation accounts for two-thirds (66–70%) of CF cases worldwide and 90% of cases in the United States; however, over 1500 other mutations can produce CF.
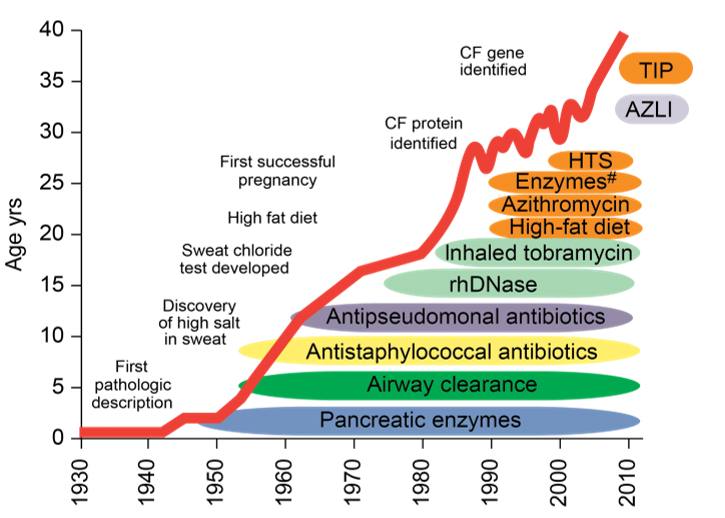
There is no known cure for Cystic Fibrosis. Lung infections are treated with antibiotics which may be given intravenously, inhaled, or orally. People with the disease take between 50-80 medications per day, and on a daily basis partake in physical therapy in order to facilitate the lungs, inhalation treatments to relieve respiratory distress, and are hospitalized every couple of months.
Over the years, many drugs have been developed to help patients cope with symptoms and increase life expectancy. However, survival age is still only 40 years, as of 2015. In other words, these people are born knowing that they will not have a long life.
Our group has focused on solving the root cause i.e. correcting the CFTR mutation. Thus, we were looking for gene editing technology.
CRISPR / Cas9
The CRISPR/Cas system is a prokaryotic immune system that confers resistance to foreign genetic elements and provides a form of acquired immunity. CRISPR associated proteins (Cas) use the CRISPR spacers to recognize and cut these exogenous genetic elements in a manner analogous to RNA interference in eukaryotic organisms.
By delivering the Cas9 nuclease and appropriate guide RNAs into a cell, the cell's genome can be cut at a desired location, allowing existing genes to be removed and/or new ones to be added by homologous recombination resulting in the correction of the CFTR mutation.
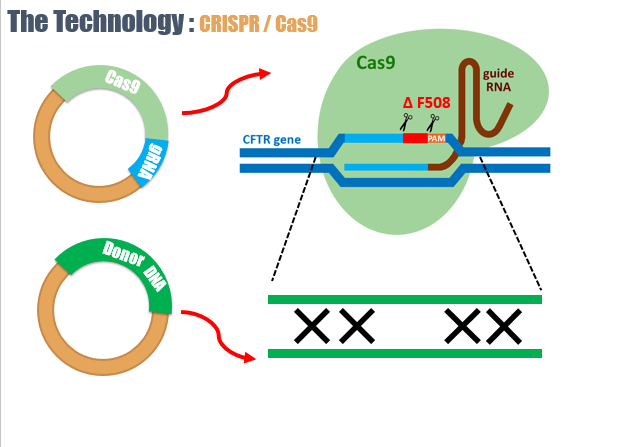
Next, our group though about a way to deliver CRISPR/Cas9 into the mutated cells.
Cholera Toxin (CT)
CT is composed of two molecular subunits: Cholera toxin subunit A (CTA) and B (CTB). CTA is responsible for the toxic effect, whereas the non-toxic subunit B is responsible for the internalization and transport of the toxin into the cell.
CTB is highly specific to epithelial cells (those are the mutated cells in CF). With its ability to bind the ganglioside receptor (GM1) upon the epithelial cell membrane, we believe it will become a successful carrier for introducing the plasmids of the CRISPR into the cells. .

In this project, we are combining these three elements together to develop a delivery system that can cure Cystic Fibrosis with a single treatment.

