m (→General objective) |
|||
| (83 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:Aix-Marseille/Template-Top|Design}} | {{:Team:Aix-Marseille/Template-Top|Design}} | ||
| − | == | + | ==General objective== |
| + | Nowadays, platinum ressources are an issue because of their planned disappearance toward 2064. Moreover, any alternative has been found so far to counteract the disparition of this metal compared to its central importance in many industries (electronic, automobile, medical..) . | ||
| − | < | + | Facing this problem, our goal here is to make a sustainable process, extracting platinum from an other source (road, sludge) than mines ressources, less pollutant, less expensive.. |
| + | |||
| + | Accumulation of the metal on the road side has been frequently shown since the beginning of the 21st century. Futhermore, alternatives methods to recycle precious metal are investigated. Here, we use the adsorption potentiel of bacterial natural componants (specifically flagellum and biopeptides) to make a process able to extract or concentrate the platinum available on different compartiments like soil, plants, sewege sludge... | ||
| + | |||
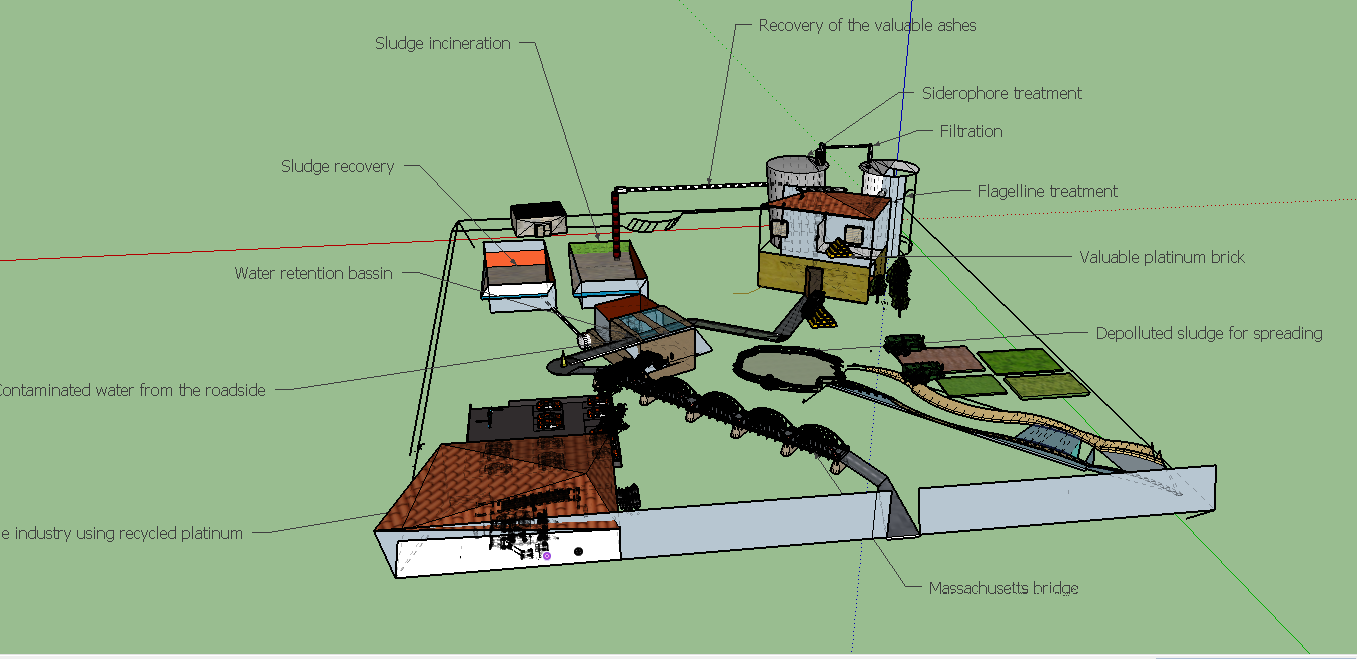
| + | [[File:T--Aix-Marseille--maquette2.jpeg|770px|center|thumb|Maquette of our process in the futur : Process has been imagined in a circular platinum economy based on the sludge treatment. Platinum found on the water retention basin is recovered and incinerated. Though, valuable ashes obtained would be treated by both steps we made : the first one is a siderophore treatment making a first concentration of the platinum and other metals, the second one is a flagellin treatment allowing to make industrialisable platinum. In consequence, treated sludge are depolluted from metal and can be spreaded on the crops making an additional valorization. The recycled platinum can be used in automobile industry for example and be an element of new automobiles. And then the platinum come back to it first form and can be recycle and the cycle begin, the cycle of platinum!. ]] | ||
| + | |||
| + | ==Source of platinum== | ||
| + | Since a law from 1993, platinum is largely used in catalytic converter to avoid toxic gaz release <ref>J. de Aberasturi, et al., Minerals Engineering 24, 505 (2011)</ref>. . During the automobile functioning, the precious metal is rejected and deposed on the road under a ionic form <ref>P.S Hooda & al. 2007, https://www.ncbi.nlm.nih.gov/pubmed/17604084</ref><ref>Liliane Michel Legret & al. http://link.springer.com/article/10.1007/s11368-012-0491-3.</ref> Platinum leaching leads to an accumulation of this metal on the road side. By default, it has been prooved that plants are potential bioaccumulator of platinum, which is found accumulated on the roots, leaves or into them. Otherwise, the metal is also carried on the sewage sludge and is actually an issue regarding the recycling potential of these sludge. | ||
| + | |||
| + | [[File:T--Aix-Marseille--platine.jpeg|500px|center|thumb|Platinum sources]] | ||
==Mobilisation by a siderophore== | ==Mobilisation by a siderophore== | ||
| − | + | In the previous article <ref> Improving recoveries of platinum and palladium from oxidized Platinum-group element ores of the Great Dyke, Zimbabwe, using the biogenic siderophore Desferrioxamine B, Dennis Kraemer & al. </ref>, authors made bioleaching with chemically synthetised siderophores. By this way, they extracted approximately 80% of the total platinum found in the ore. But we were looking for a synthetic biological approach. A biological approach can lead to an easier and cheaper way to extract minerals. | |
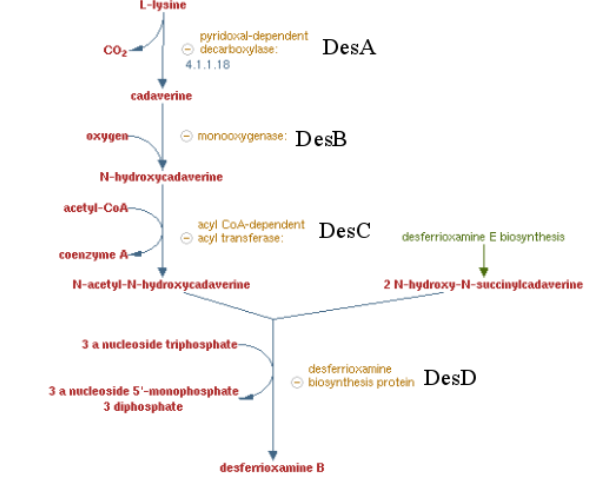
| − | + | ===Pathway of the desferrioxamine B biosynthesis=== | |
| + | [[File:T--Aix-Marseille--desABCD.jpeg|600px|center|thumb|Desferrioxamine B pathway]] | ||
| − | + | Previous work showed that siderophores which are molecule secreted by bacteria to catch iron are able to catch other metals by default. Specifically, many articles showed a high affinity of Desferrioxamine B ( produce by ''Streptomyces coelicolor'') for tetravalent metal ions and more specifically platinum. These results encouraged us to make a biobrick coding the sequence corresponding to the 4 enzymes involved on the metabolic pathway of Desferrioxamine B. <i>E. coli</i> was used to produce this biobrick. Produce a gram positive bacteria pathway in a gram negative bacteria is restrictive <ref> Wandersman & Delepaire https://www.ncbi.nlm.nih.gov/pubmed/15487950 </ref> , considering the risk of toxicity for the conductress bacteria. To counter this potential issue, we regulate transcription using the arabinose inductible promoter, pBAD/araC | |
| − | + | It has to be noted that the siderophore here is specific for platinum but the same cloning could be performed with siderophores specific for others metals, thus our process presents a great potential as it can be applied to others metals thanks to synthetic biology and its modularity. | |
| − | + | ===Our strategy=== | |
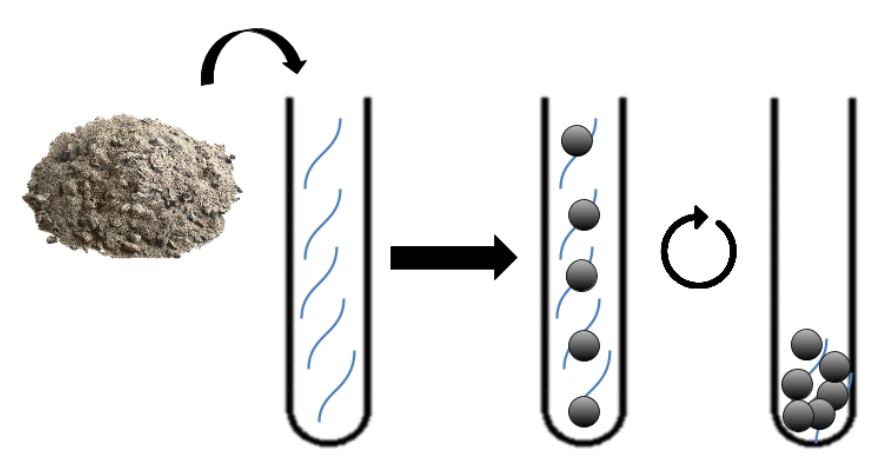
| − | Our strategy for the siderophore mobilisation results in two main steps : | + | Our strategy for the siderophore mobilisation results in two main steps : |
| − | + | ||
| − | + | ||
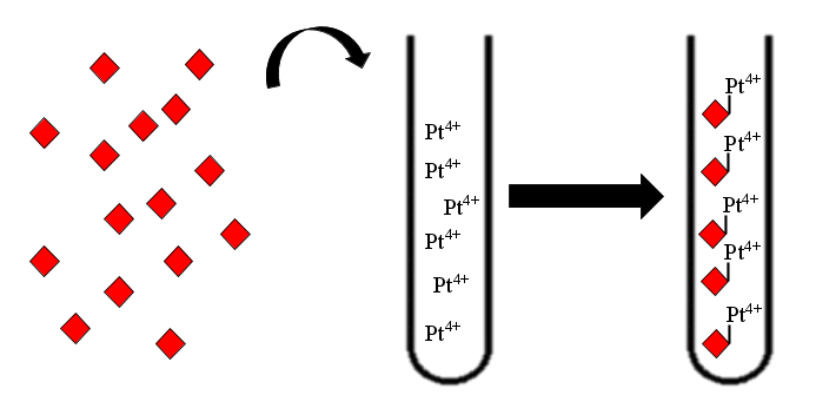
| − | + | [[File:T--Aix-Marseille--siderophore_binding.jpeg|200px|left|thumb|Siderophores binding the platinum ions and others metallic ions]] | |
| − | + | [[File:T--Aix-Marseille--siderophore_pathway.jpeg|200px|right|thumb|Siderophore production strategy]] | |
| + | |||
| + | 1. Transformation of E.Coli with an inductible plasmide holding the ''des'' cluster. | ||
| − | + | 2. Induction and production of the desferrioxamine B. | |
| + | |||
| + | |||
| + | |||
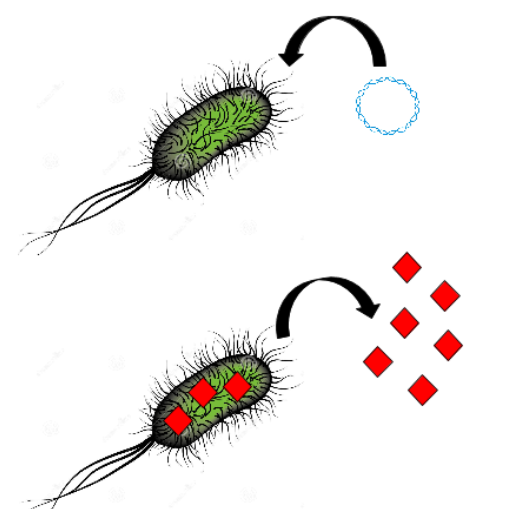
| + | Then, the produced siderophore would be purified and transfered in the recuperation solution containing the platinum and other metals. Therefore, those metals must be recruited by our purified siderophores. Though those siderophores would be import using a sowing of ''Streptomyces coelicolor''. Then simple centrifugation and combustion will concentrate the solution in metal a first time. | ||
| + | [[File:T--Aix-Marseille--streptomyces.jpeg|300px|center|thumb|Importation of the siderophore complexed with the platinum in Streptomyces]] | ||
==Biosorption and reduction using flagellin and peptides == | ==Biosorption and reduction using flagellin and peptides == | ||
| − | < | + | |
| − | + | ===Our references=== | |
| + | |||
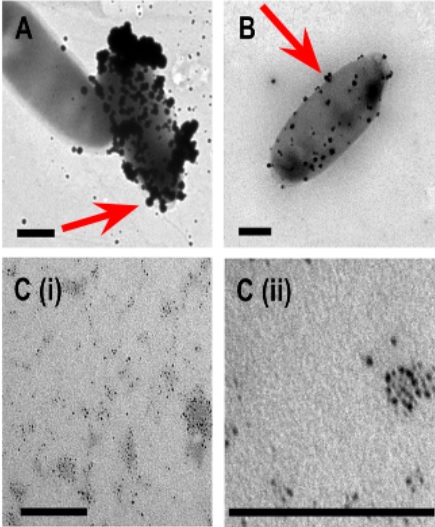
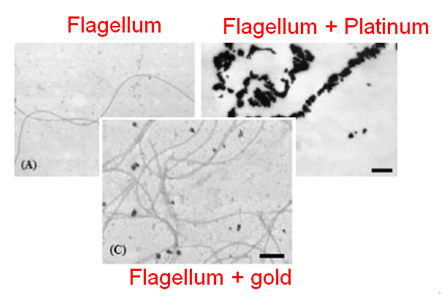
| + | [[File:T--Aix-Marseille--Flagel_agregat.jpeg|200px|right|thumb|Adsorption of the platinum on the bacterial surface, Capeness & al. 2015]] | ||
| + | [[File:Biosfhsdh.png|200px|left|thumb|Adsorption of the platinum on the flagellum, Deplenche & al. 2008]] | ||
| + | |||
| + | It was shown that proteins are able to bind platinium and especially bacterial flagellums which are naturally a high platinum adsorber <ref>Capeness & al. 2015, http://eprints.nottingham.ac.uk/27979/1/Michael%20Capeness%20-%20Thesis%20-%20PDF.pdf</ref><ref>Deplanche & al., 2007 http://onlinelibrary.wiley.com/doi/10.1002/bit.21688/abstract. </ref> Furthermore, many peptides were generated based on sequences selected by phage display in order to enhance metal ions adsorption including gold, silver, platinum and a plenty of other metals. This enhances the potential of our process which can be performed with other metals.<ref>Uratu Ozgur Safak Seker & al., 2011, http://www.mdpi.com/1420-3049/16/2/1426.</ref> | ||
| + | |||
| + | === Our strategy === | ||
This step aims to : | This step aims to : | ||
| − | + | * adsorb ions on bacterial flagella protein | |
| − | + | * reduce ions into reduced nanoparticles by the ambient reducer power | |
| − | + | All together, these findings incite us to use these natural properties to build a biobrick which is a high affinity binder of platinum based on <i>E. coli</i> and <i>Desulfovibrio vulgaris</i> flagellum and synthetic peptides. To this end, we analyze the flagella sequences and structural properties of the external part of the flagella. Then, on the part of the flagellin facing the external medium, an insertion restriction site will be inserted. Then specific precious metal peptides would be added using this insertion site to increase the level of adsorption specificity and yield. In this way, peptide would be facing the external medium and being able to bind metallic ions. To obtain a high transcription level of this sequence, we put transcription control under a strong promoter enabling a high flagellin production. | |
| − | </ | + | [[File:T--Aix-Marseille--flagel_captation.jpeg|500px|center|thumb|Adsorption of the platinum on the flagellin]] |
| − | < | + | Next, the flagellin produced will be added to the first concentrated platinum solution. Flagellin containing specific peptides will bind the free or available ions in the medium and reduce them into reduced nanoparticules usable in industry. A simple centrifugation of the flagellin binding the platinum allows to concentrate the metal a second time. |
| + | |||
| + | <references/> | ||
| − | + | {{:Team:Aix-Marseille/Template-Footer}} | |
| − | + | ||
Design
General objective
Nowadays, platinum ressources are an issue because of their planned disappearance toward 2064. Moreover, any alternative has been found so far to counteract the disparition of this metal compared to its central importance in many industries (electronic, automobile, medical..) .
Facing this problem, our goal here is to make a sustainable process, extracting platinum from an other source (road, sludge) than mines ressources, less pollutant, less expensive..
Accumulation of the metal on the road side has been frequently shown since the beginning of the 21st century. Futhermore, alternatives methods to recycle precious metal are investigated. Here, we use the adsorption potentiel of bacterial natural componants (specifically flagellum and biopeptides) to make a process able to extract or concentrate the platinum available on different compartiments like soil, plants, sewege sludge...
Source of platinum
Since a law from 1993, platinum is largely used in catalytic converter to avoid toxic gaz release [1]. . During the automobile functioning, the precious metal is rejected and deposed on the road under a ionic form [2][3] Platinum leaching leads to an accumulation of this metal on the road side. By default, it has been prooved that plants are potential bioaccumulator of platinum, which is found accumulated on the roots, leaves or into them. Otherwise, the metal is also carried on the sewage sludge and is actually an issue regarding the recycling potential of these sludge.
Mobilisation by a siderophore
In the previous article [4], authors made bioleaching with chemically synthetised siderophores. By this way, they extracted approximately 80% of the total platinum found in the ore. But we were looking for a synthetic biological approach. A biological approach can lead to an easier and cheaper way to extract minerals.
Pathway of the desferrioxamine B biosynthesis
Previous work showed that siderophores which are molecule secreted by bacteria to catch iron are able to catch other metals by default. Specifically, many articles showed a high affinity of Desferrioxamine B ( produce by Streptomyces coelicolor) for tetravalent metal ions and more specifically platinum. These results encouraged us to make a biobrick coding the sequence corresponding to the 4 enzymes involved on the metabolic pathway of Desferrioxamine B. E. coli was used to produce this biobrick. Produce a gram positive bacteria pathway in a gram negative bacteria is restrictive [5] , considering the risk of toxicity for the conductress bacteria. To counter this potential issue, we regulate transcription using the arabinose inductible promoter, pBAD/araC
It has to be noted that the siderophore here is specific for platinum but the same cloning could be performed with siderophores specific for others metals, thus our process presents a great potential as it can be applied to others metals thanks to synthetic biology and its modularity.
Our strategy
Our strategy for the siderophore mobilisation results in two main steps :
1. Transformation of E.Coli with an inductible plasmide holding the des cluster.
2. Induction and production of the desferrioxamine B.
Then, the produced siderophore would be purified and transfered in the recuperation solution containing the platinum and other metals. Therefore, those metals must be recruited by our purified siderophores. Though those siderophores would be import using a sowing of Streptomyces coelicolor. Then simple centrifugation and combustion will concentrate the solution in metal a first time.
Biosorption and reduction using flagellin and peptides
Our references
It was shown that proteins are able to bind platinium and especially bacterial flagellums which are naturally a high platinum adsorber [6][7] Furthermore, many peptides were generated based on sequences selected by phage display in order to enhance metal ions adsorption including gold, silver, platinum and a plenty of other metals. This enhances the potential of our process which can be performed with other metals.[8]
Our strategy
This step aims to :
- adsorb ions on bacterial flagella protein
- reduce ions into reduced nanoparticles by the ambient reducer power
All together, these findings incite us to use these natural properties to build a biobrick which is a high affinity binder of platinum based on E. coli and Desulfovibrio vulgaris flagellum and synthetic peptides. To this end, we analyze the flagella sequences and structural properties of the external part of the flagella. Then, on the part of the flagellin facing the external medium, an insertion restriction site will be inserted. Then specific precious metal peptides would be added using this insertion site to increase the level of adsorption specificity and yield. In this way, peptide would be facing the external medium and being able to bind metallic ions. To obtain a high transcription level of this sequence, we put transcription control under a strong promoter enabling a high flagellin production.
Next, the flagellin produced will be added to the first concentrated platinum solution. Flagellin containing specific peptides will bind the free or available ions in the medium and reduce them into reduced nanoparticules usable in industry. A simple centrifugation of the flagellin binding the platinum allows to concentrate the metal a second time.
- ↑ J. de Aberasturi, et al., Minerals Engineering 24, 505 (2011)
- ↑ P.S Hooda & al. 2007, https://www.ncbi.nlm.nih.gov/pubmed/17604084
- ↑ Liliane Michel Legret & al. http://link.springer.com/article/10.1007/s11368-012-0491-3.
- ↑ Improving recoveries of platinum and palladium from oxidized Platinum-group element ores of the Great Dyke, Zimbabwe, using the biogenic siderophore Desferrioxamine B, Dennis Kraemer & al.
- ↑ Wandersman & Delepaire https://www.ncbi.nlm.nih.gov/pubmed/15487950
- ↑ Capeness & al. 2015, http://eprints.nottingham.ac.uk/27979/1/Michael%20Capeness%20-%20Thesis%20-%20PDF.pdf
- ↑ Deplanche & al., 2007 http://onlinelibrary.wiley.com/doi/10.1002/bit.21688/abstract.
- ↑ Uratu Ozgur Safak Seker & al., 2011, http://www.mdpi.com/1420-3049/16/2/1426.