m (→Biotechnological solutions) |
|||
| (67 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{:Team:Aix-Marseille/Template-Top| | + | {{:Team:Aix-Marseille/Template-Top|Platinum in the environment}} |
| − | + | #[[Team:Aix-Marseille/Integrated_Practices/history|Metals importance throughout history]] | |
| + | #[[Team:Aix-Marseille/Integrated_Practices/Mines|Platinum in mines]] | ||
| + | #[[Team:Aix-Marseille/Integrated_Practices/Industry|Platinum in industry]] | ||
| + | #[[Team:Aix-Marseille/Integrated_Practices/Environment|Platinum in the environment]] | ||
| + | #[[Team:Aix-Marseille/Integrated_Practices/Process|Our process]] | ||
| − | + | '''Thoughts and content displayed on this page have been elaborated thank to the help of Frédéric Triboit an ecologist engineer, PhD in Environmental Sciences. He helped us a lot in the targeting to choose which raw materials would be the source of our process and also to understand the critical situation faced today with metal pollution in environment especially along roadsides.''' | |
| − | == | + | ==Situation== |
| − | + | ===The Source=== | |
| + | [[File:T--Aix-Marseille--routes.jpg|thumb|400px|right|The car traffic is the main source of platinum pollution all over the world, especially along the sidesroads]] | ||
| + | The main source is obviously the catalytic converters. As a catalyst platinum is not supposed to leave the converter but during the lifetime of the converter a certain amount is released and is dispersed in environment. [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Industry Others uses] of platinum can generate pollution with platinum as the uses in the medical field. Patients will be exposed at high concentration and will reject (feces and urine) in sewage high amounts of platinum. All the electronic industry is a source of pollution, since the e-waste are enriched in platinum. If recycling is not properly performed this platinum can be released in environment. | ||
| − | + | A plenty of studies assess the increase in metals around in environment. Many places and many metals are concerned all over the world and to demonstrate that, representative example are displayed in this page. Metal pollution in environment is diverse and platinum is not the only metal polluting the environment, not at all. However we'll focus here on Platinum and Palladium pollution. | |
| − | + | ===Platinum along roads=== | |
| − | + | ||
| − | + | As platinum and palladium are used as catalytic converters the main source of pollution is close the axis of car traffic, i.e. roads and highways. Several studies show different concentrations as context of measures are always different and the factors involved in concentration are much complex. | |
| − | + | The average concentration of platinum and palladium can reach values of '''22.74µg/kg''' for platinum and '''120.8µg/kg''' just near the road. As we can foresee, the concentration is dependent of the distance of the road and indeed values fall to 2.04µg/kg and 84.2µg/kg for platinum and palladium respectively is measure are carried out 5 meters away from the road <ref name="hooda2007">http://www.sciencedirect.com/science/article/pii/S0048969707006390 Hooda et al., 2007 </ref> | |
| − | + | ||
| − | + | Near roads in Germany, with a heavy traffic of about 16,000 car a days and with a speed limit of 80km/h, platinum concentrations have been estimated around '''50µg/kg'''. | |
| − | + | Even higher concentrations have been found, reaching the impressive value of '''132µg/kg'''<ref>http://pubs.acs.org/doi/abs/10.1021/es061453s Zereini and al., 2007</ref>. | |
| − | == | + | ===Platinum in plants=== |
| − | + | Plants are a biological '''compartment''' able to '''concentrate''' all type of pollutants present in the environment. Thus, many studies have been carried out to assess the concentration in plants living near pollution source as a highway or a road. | |
| − | + | ||
| − | + | Plants thriving near the roads '''accumulate metals''' as expected as their concentrations for platinum and palladium respectively are in average about '''8.64µg/kg''' and '''10µg/kg''' respectively<ref name="hooda2007"/>. | |
| + | Some species of birch trees are also able to accumulate platinum to a value up to '''1350ng/g''' and ashes from pine trees presents platinum concentration around '''5000ng/g'''<ref name="reith2014">http://www.sciencedirect.com/science/article/pii/S0012825214000063?np=y Reith and al., 2014</ref>. | ||
| + | ===Platinum in rivers=== | ||
| − | + | [[File:T--Aix-Marseille--Rhine.jpg|thumb|400px|left|The Rhine river, a major axis of industry and transport in Europe thus strongly exposed to platinum and metals pollution]] | |
| − | + | As rivers collect all stream and watercourses, all the washed pollutants are gathered in rivers, especially after a phenomenon of washing i.e. rain. | |
| − | + | Concentrations found in Rhine river help to realize how concentrations in platinum could increase with the effluents due to the rain washing. Concentration usually found are about '''5ng/g''' but after a rain events (all soils, and surfaces are washed out) this concentration reach a value of '''31.2µg/g''' thus increased a '''thousand''' times <ref name="reith2014"/> | |
| − | + | ===Platinum in urban area=== | |
| − | + | ||
| − | + | In urban area, the source of platinum are everywhere as many types of vehicles are always present in thoses places. Exhaust gas from catalyst converter are pervasive in such area and scientist have expected to find high, hence their studies in those areas. | |
| − | = | + | Concentrations in soils town center have even shown a value of '''90µg/kg'''!<ref name="wichmann2007">http://www.sciencedirect.com/science/article/pii/S0048969707007905 Wichmann and al., 2007</ref> |
| − | + | ||
| − | + | Much higher value have been measured in air born dust, with a mean of '''1730µg/kg'''<ref name="wichmann2007"/>. | |
| − | == | + | ===Platinum in sewage sludge=== |
| − | + | If rivers gather pollution, the sewage sludge contain the main pollutants once presents in the waters.. | |
| + | In sewage sludge,one of the most promising start for our process, all the residuals metals concentrate especially after rain events. | ||
| + | Concentration have been measured around '''602µg/kg''' for platinum and 722 µg/kg''' for palladium<ref>https://www.ncbi.nlm.nih.gov/pubmed/19878972 Jackson, Prichard and Sampson., 2010</ref>. | ||
| + | As produced steadily in great quantities, sewage sludge could be a very good source for our process. | ||
| + | |||
| + | ===Average guide platinum concentrations=== | ||
| + | In earth crust :'''5µg/kg''' | ||
| + | In coal: '''5µg/kg''' | ||
| + | In mined fields: '''1500µg/kg''' | ||
| + | In your blood : '''0.1- 3.8µg/kg''' | ||
| + | |||
| + | ==Classical solutions== | ||
| + | |||
| + | Metals pollution in environment is a concern for health authorities for a long time. However metal pollution is increasing '''so fast''' for '''so many metals''' (Pt, Pd, Rh, Co, Pb, Mo, Cu, Zn, Hg, Ni, Sb, Se...) that the actual solution to deal with such pollution is actually to burn and confine ashes from sewage sludge. Indeed the classical treatment with '''bacteria is not efficient''' to eliminate such metals pollution. So the sludge are burned and then stocked as pollutant materials. | ||
| + | |||
| + | This solution allow to prevent the contamination in environment since sludge are classically spread in field, but it doesn't reduce pollution in environment and worse, produce '''contaminated ashes''', unvaluable and unusable materials which need to be '''stocked'''. | ||
| + | |||
| + | ==Biotechnological solutions== | ||
| + | [[File:T--Aix-Marseille--frf.jpg|thumb|right|500px| ''Pycnandra acuminata'' is able to accumulate Nickel up to 20% of the weigh of its life blood hence its blue color <ref>Source: B. Fogliani-LIVE-UNC</ref>]] | ||
| + | The '''phytoremediation''' use plants and their ability to concentrate or destroy pollutant to clean up soils or waters. | ||
| + | Plants thriving along the roads are continuously exposed to the metals pollution, so they can be used as a '''compartments''' in order to '''remove pollutants'''. | ||
| + | |||
| + | Some plant species are well known to accumulate metals as ''Pycnandra acuminata'', see photo. Metals accumulation are sometimes so powerful, that techniques known as '''Biomining''' are planned to be used: these plants could even be an '''alternative''' to classical mining. Since plants are naturally growing along the roadsides and are currently cut off regularly (but not used!) this technique doesn't involve a lot of change in actual proceeding along the roadsides. | ||
| + | |||
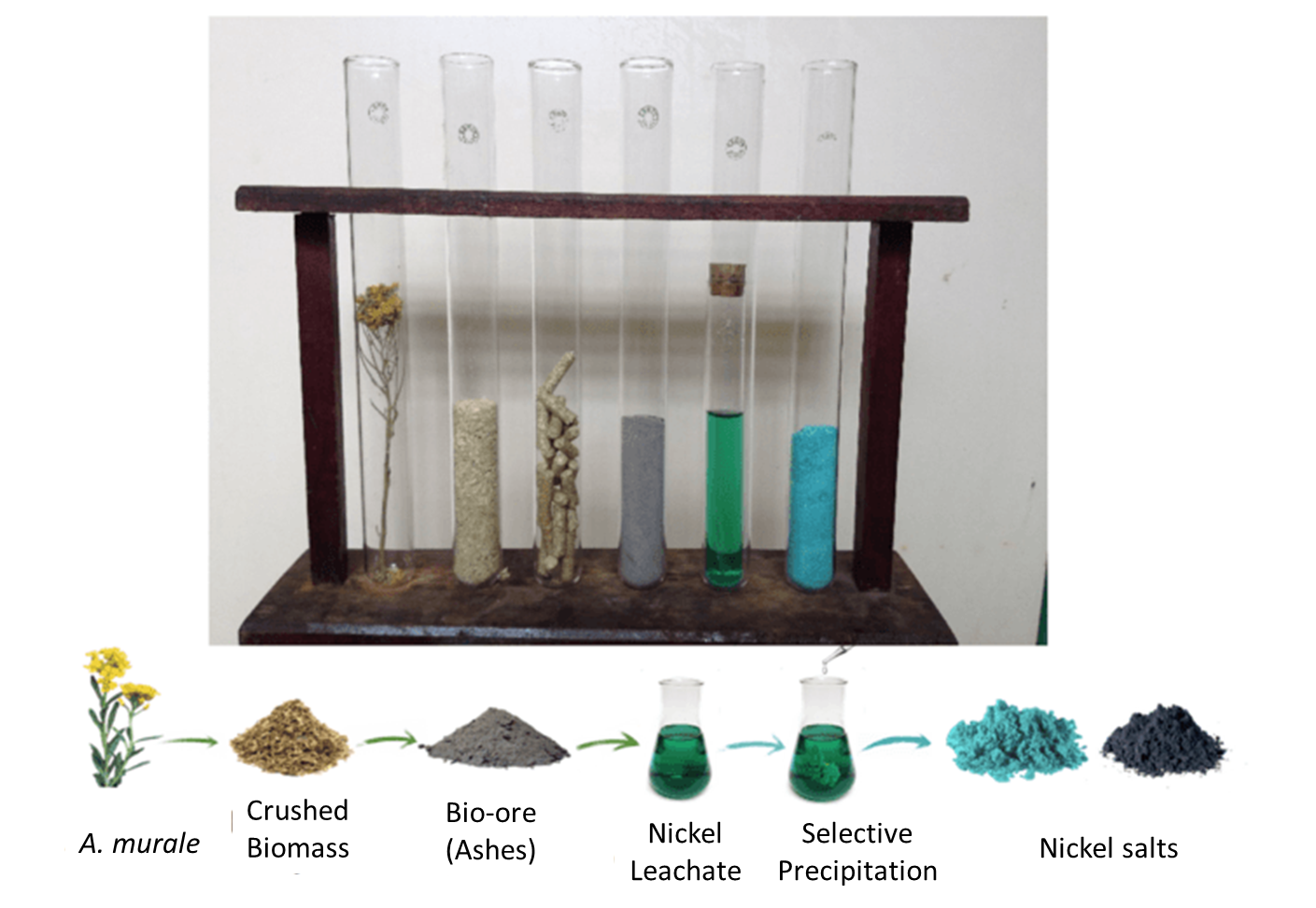
| + | If all the gathered cut grass is incinerated, treatment processes starting from the ashes could remove metals, like [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Process our process]. Such processes are currently being developed as for ''Alyssum murale '' a plant able to accumulate Nickel too. | ||
| + | [[File:T--Aix-Marseille--nickel_mining.png|thumb|500px|left|''Alyssum murale'' is a plant able to perform biomining on nickel. Techniques are being developed in laboratory to process the ashes and remove metals<ref>Author: Baptiste Laubie, Laboratory LGPB, CNRS</ref>]] | ||
| + | |||
| + | The advantage of phytoremediation is that this process rely on the uses of biomass, and biomass is produced easily almost everywhere. Biomass can be used for another purpose before being used to recover precious metals as platinum or to remove from the environment toxic and pollutant metals. | ||
| + | Process of '''methanization''' with such green biomass can be achieved in order to produce biogas. Then the '''digestate''' can enter a process of treatment... | ||
| + | '''Composting''', a widespread practice, could also provide '''compostate''' to supply the treatment processes. As most of the plants have been exposed to metals pollution during their growth, all green biomass are candidate to enter the treatment processes. | ||
| + | |||
| + | Techniques using '''phytoremediation''' in sewage sludge treatment are also developed. Plants can be grown in treatment pools to concentrate pollutants or to remove pollutants by '''phytovolatilization''' : pollutants are not concentrated within plants but are "sprayed" in the air by the plants. | ||
| + | |||
| + | So a very large range of actual techniques can be used to remove pollutants. Many solutions exist and they often provide a way to clean up the environment but also a way to recycle biomass compost heap, produce energy and gas (methanization)... | ||
| + | All these bio-ecological solutions could be performed together and would lead to far better situations than we have today! | ||
| + | |||
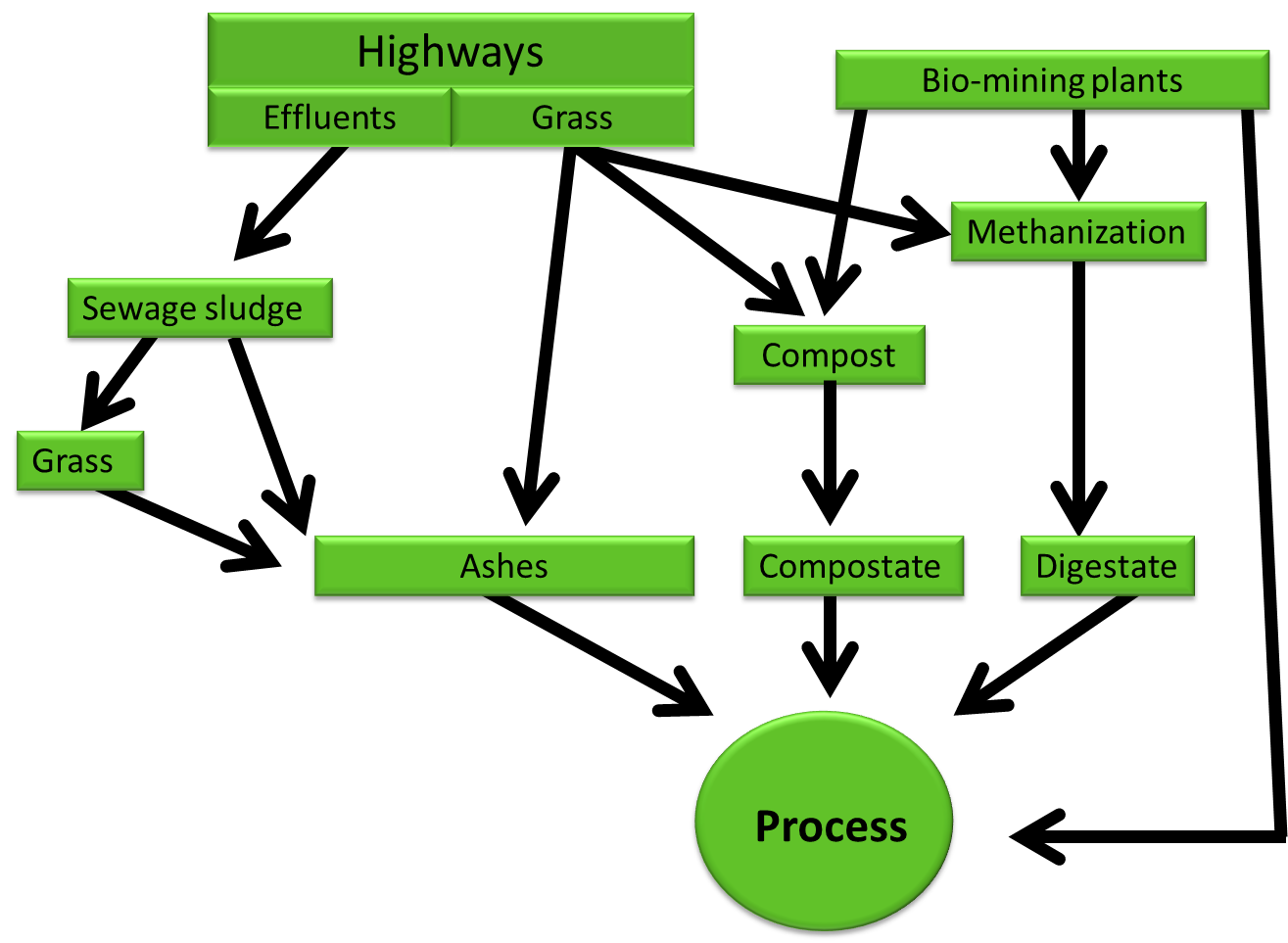
| + | All these techniques provides raw materials as '''ashes''', '''compostate''', '''digestate''' that '''could be a start to many further processes''' designed to enhance recovery or cleaning up, depending on metals nature. | ||
| + | '''This is precisely where our project could start''' (see figure). The advantage is to start with raw materials and switch in a controlled environment, see the details of [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Process our process in industry]. | ||
| + | |||
| + | [[File:T--Aix-Marseille--essaideschema3.png|thumb|800px|center|Potential connection between a some available biotechnological techniques. The final process could be [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Process ours] '''As displayed, our process is able to accept different raw materials, from several process realized upstream.''']] | ||
| + | |||
| + | |||
| + | '''Again we are thankful to Frederic Triboit to have helped us to shape our project thanks to his wise and farsighted explanations and advice about current metal pollution.''' | ||
| + | |||
| + | |||
| + | |||
| + | <references/> | ||
{{:Team:Aix-Marseille/Template-Footer}} | {{:Team:Aix-Marseille/Template-Footer}} | ||
Platinum in the environment
- Metals importance throughout history
- Platinum in mines
- Platinum in industry
- Platinum in the environment
- Our process
Thoughts and content displayed on this page have been elaborated thank to the help of Frédéric Triboit an ecologist engineer, PhD in Environmental Sciences. He helped us a lot in the targeting to choose which raw materials would be the source of our process and also to understand the critical situation faced today with metal pollution in environment especially along roadsides.
Situation
The Source
The main source is obviously the catalytic converters. As a catalyst platinum is not supposed to leave the converter but during the lifetime of the converter a certain amount is released and is dispersed in environment. Others uses of platinum can generate pollution with platinum as the uses in the medical field. Patients will be exposed at high concentration and will reject (feces and urine) in sewage high amounts of platinum. All the electronic industry is a source of pollution, since the e-waste are enriched in platinum. If recycling is not properly performed this platinum can be released in environment.
A plenty of studies assess the increase in metals around in environment. Many places and many metals are concerned all over the world and to demonstrate that, representative example are displayed in this page. Metal pollution in environment is diverse and platinum is not the only metal polluting the environment, not at all. However we'll focus here on Platinum and Palladium pollution.
Platinum along roads
As platinum and palladium are used as catalytic converters the main source of pollution is close the axis of car traffic, i.e. roads and highways. Several studies show different concentrations as context of measures are always different and the factors involved in concentration are much complex.
The average concentration of platinum and palladium can reach values of 22.74µg/kg for platinum and 120.8µg/kg just near the road. As we can foresee, the concentration is dependent of the distance of the road and indeed values fall to 2.04µg/kg and 84.2µg/kg for platinum and palladium respectively is measure are carried out 5 meters away from the road [1]
Near roads in Germany, with a heavy traffic of about 16,000 car a days and with a speed limit of 80km/h, platinum concentrations have been estimated around 50µg/kg. Even higher concentrations have been found, reaching the impressive value of 132µg/kg[2].
Platinum in plants
Plants are a biological compartment able to concentrate all type of pollutants present in the environment. Thus, many studies have been carried out to assess the concentration in plants living near pollution source as a highway or a road.
Plants thriving near the roads accumulate metals as expected as their concentrations for platinum and palladium respectively are in average about 8.64µg/kg and 10µg/kg respectively[1]. Some species of birch trees are also able to accumulate platinum to a value up to 1350ng/g and ashes from pine trees presents platinum concentration around 5000ng/g[3].
Platinum in rivers
As rivers collect all stream and watercourses, all the washed pollutants are gathered in rivers, especially after a phenomenon of washing i.e. rain. Concentrations found in Rhine river help to realize how concentrations in platinum could increase with the effluents due to the rain washing. Concentration usually found are about 5ng/g but after a rain events (all soils, and surfaces are washed out) this concentration reach a value of 31.2µg/g thus increased a thousand times [3]
Platinum in urban area
In urban area, the source of platinum are everywhere as many types of vehicles are always present in thoses places. Exhaust gas from catalyst converter are pervasive in such area and scientist have expected to find high, hence their studies in those areas.
Concentrations in soils town center have even shown a value of 90µg/kg![4]
Much higher value have been measured in air born dust, with a mean of 1730µg/kg[4].
Platinum in sewage sludge
If rivers gather pollution, the sewage sludge contain the main pollutants once presents in the waters.. In sewage sludge,one of the most promising start for our process, all the residuals metals concentrate especially after rain events. Concentration have been measured around 602µg/kg for platinum and 722 µg/kg for palladium[5]. As produced steadily in great quantities, sewage sludge could be a very good source for our process.
Average guide platinum concentrations
In earth crust :5µg/kg In coal: 5µg/kg In mined fields: 1500µg/kg In your blood : 0.1- 3.8µg/kg
Classical solutions
Metals pollution in environment is a concern for health authorities for a long time. However metal pollution is increasing so fast for so many metals (Pt, Pd, Rh, Co, Pb, Mo, Cu, Zn, Hg, Ni, Sb, Se...) that the actual solution to deal with such pollution is actually to burn and confine ashes from sewage sludge. Indeed the classical treatment with bacteria is not efficient to eliminate such metals pollution. So the sludge are burned and then stocked as pollutant materials.
This solution allow to prevent the contamination in environment since sludge are classically spread in field, but it doesn't reduce pollution in environment and worse, produce contaminated ashes, unvaluable and unusable materials which need to be stocked.
Biotechnological solutions

The phytoremediation use plants and their ability to concentrate or destroy pollutant to clean up soils or waters. Plants thriving along the roads are continuously exposed to the metals pollution, so they can be used as a compartments in order to remove pollutants.
Some plant species are well known to accumulate metals as Pycnandra acuminata, see photo. Metals accumulation are sometimes so powerful, that techniques known as Biomining are planned to be used: these plants could even be an alternative to classical mining. Since plants are naturally growing along the roadsides and are currently cut off regularly (but not used!) this technique doesn't involve a lot of change in actual proceeding along the roadsides.
If all the gathered cut grass is incinerated, treatment processes starting from the ashes could remove metals, like our process. Such processes are currently being developed as for Alyssum murale a plant able to accumulate Nickel too.
The advantage of phytoremediation is that this process rely on the uses of biomass, and biomass is produced easily almost everywhere. Biomass can be used for another purpose before being used to recover precious metals as platinum or to remove from the environment toxic and pollutant metals. Process of methanization with such green biomass can be achieved in order to produce biogas. Then the digestate can enter a process of treatment... Composting, a widespread practice, could also provide compostate to supply the treatment processes. As most of the plants have been exposed to metals pollution during their growth, all green biomass are candidate to enter the treatment processes.
Techniques using phytoremediation in sewage sludge treatment are also developed. Plants can be grown in treatment pools to concentrate pollutants or to remove pollutants by phytovolatilization : pollutants are not concentrated within plants but are "sprayed" in the air by the plants.
So a very large range of actual techniques can be used to remove pollutants. Many solutions exist and they often provide a way to clean up the environment but also a way to recycle biomass compost heap, produce energy and gas (methanization)... All these bio-ecological solutions could be performed together and would lead to far better situations than we have today!
All these techniques provides raw materials as ashes, compostate, digestate that could be a start to many further processes designed to enhance recovery or cleaning up, depending on metals nature. This is precisely where our project could start (see figure). The advantage is to start with raw materials and switch in a controlled environment, see the details of our process in industry.
Again we are thankful to Frederic Triboit to have helped us to shape our project thanks to his wise and farsighted explanations and advice about current metal pollution.
- ↑ 1.0 1.1 http://www.sciencedirect.com/science/article/pii/S0048969707006390 Hooda et al., 2007
- ↑ http://pubs.acs.org/doi/abs/10.1021/es061453s Zereini and al., 2007
- ↑ 3.0 3.1 http://www.sciencedirect.com/science/article/pii/S0012825214000063?np=y Reith and al., 2014
- ↑ 4.0 4.1 http://www.sciencedirect.com/science/article/pii/S0048969707007905 Wichmann and al., 2007
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/19878972 Jackson, Prichard and Sampson., 2010
- ↑ Source: B. Fogliani-LIVE-UNC
- ↑ Author: Baptiste Laubie, Laboratory LGPB, CNRS



