| (22 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{:Team:Aix-Marseille/Template-Top|Our process}} | + | {{:Team:Aix-Marseille/Template-Top|Our process}} |
| − | + | #[[Team:Aix-Marseille/Integrated_Practices/history|Metals importance throughout history]] | |
| + | #[[Team:Aix-Marseille/Integrated_Practices/Mines|Platinum in mines]] | ||
| + | #[[Team:Aix-Marseille/Integrated_Practices/Industry|Platinum in industry]] | ||
| + | #[[Team:Aix-Marseille/Integrated_Practices/Environment|Platinum in the environment]] | ||
| + | #[[Team:Aix-Marseille/Integrated_Practices/Process|Our process]] | ||
| + | In this page we developed all estimation and reflexion we made around our process. Thanks to Pr. Sigoillot from the INRA (The French National Institute of Agronomy) we have been able to carry out a relevant process engineering study around application our process could permit. | ||
| − | <html>< | + | <html><iframe width="560" height="315" src="https://www.youtube.com/embed/Hx-6EPR5es0" frameborder="0" allowfullscreen></iframe></html> |
| − | For the detailed interview with Pr.Sigoillot see [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Process/interview here]. | + | |
| + | '''For the detailed interview with Pr.Sigoillot see [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Process/interview here]'''. | ||
==Our Process is applicable on many ways== | ==Our Process is applicable on many ways== | ||
| Line 17: | Line 23: | ||
In this part we ll'detail an industrialized process of what we plan to test in lab condition. We are thankfull to Mr.Sigoillot, who advised us wisely in the choice of which process could be more developed in particular, see the interview [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Process/interview here]. | In this part we ll'detail an industrialized process of what we plan to test in lab condition. We are thankfull to Mr.Sigoillot, who advised us wisely in the choice of which process could be more developed in particular, see the interview [https://2016.igem.org/Team:Aix-Marseille/Integrated_Practices/Process/interview here]. | ||
| − | We'll focus on a process started from sewage sludge. Indeed, choosing sewage sludge as a source of platinum bring another benefit: it will remove platinum from sludges. Nowadays, '''sludge are so concentrated in metals that it can't be spread on field for valorisation''' and as a result, sludge are often burnt and ashes are kept in specialized confinement centers, thus making the treatment very expensive and not sustainable. So our process can remove metals aiming to recover it and doing so our process will also achieve the purpose to rid metals from sludges. Moreover, sewage sludge treatment is a payed service by institutions who needs to get rid of it (municipality, motorway operating...) so with this source of platinum our process will start with an already positive financial balance! Of courses polluting metals, preventing spreading in field are many and our process is currently designed to recover specifically and only platinum. But our process is extremely versatile and each step of our process can be modified to be specific of another metal. | + | We'll focus on a process started from sewage sludge. Indeed, choosing sewage sludge as a source of platinum bring another benefit: it will remove platinum from sludges. Nowadays, '''sludge are so concentrated in metals that it can't be spread on field for valorisation''' and as a result, sludge are often burnt and ashes are kept in specialized confinement centers, thus making the treatment very expensive and not sustainable. So our process can remove metals aiming to recover it and doing so our process will also achieve the purpose to rid metals from sludges. Moreover, sewage sludge treatment is a payed service by institutions who needs to get rid of it (municipality, motorway operating...) so with this source of platinum our process will start with an already positive financial balance! Of courses polluting metals, preventing spreading in field are many and our process is currently designed to recover specifically and only platinum. But our process is extremely versatile and each step of our process can be modified to be specific of another metal (see video below). |
| − | + | ||
| + | <html><video width="480" controls src="https://static.igem.org/mediawiki/2016/6/6b/T--Aix-Marseille--Processinterview7.mp4"></html> | ||
As you will discover in the process, [[Team:Aix-Marseille/Integrated_Practices/Process#Siderophore_mediated_leaching| step 4]] involves a [https://2016.igem.org/Team:Aix-Marseille/Design#Mobilisation_by_a_siderophore siderophore] that could be specific to another metal. Likewise, the biosorption occurred in the [[Team:Aix-Marseille/Integrated_Practices/Process#Biosorption|step 9]] involves [https://2016.igem.org/Team:Aix-Marseille/Design#Biosorption_and_reduction_using_flagellin_and_peptides small metals catching peptids], that can be used to catch far more other metals that just only platinum. | As you will discover in the process, [[Team:Aix-Marseille/Integrated_Practices/Process#Siderophore_mediated_leaching| step 4]] involves a [https://2016.igem.org/Team:Aix-Marseille/Design#Mobilisation_by_a_siderophore siderophore] that could be specific to another metal. Likewise, the biosorption occurred in the [[Team:Aix-Marseille/Integrated_Practices/Process#Biosorption|step 9]] involves [https://2016.igem.org/Team:Aix-Marseille/Design#Biosorption_and_reduction_using_flagellin_and_peptides small metals catching peptids], that can be used to catch far more other metals that just only platinum. | ||
| Line 28: | Line 35: | ||
Sewage sludge are obtained daily in high amounts, all over the world, as effluents treatment is obviously a continued process. Indeed this source is '''abondant''' and '''inexhaustible''', as in almost every city around the world, effluents are treated and sewage sludges are collected. In most of the cases, when metals concentrations in sludges are too high to perform a valorization in the environnement as spreading on fields, the procedure is to stock sludges in a confined place. To reduce the stock volume, sludges are burnt.This step is precisely where our process could be connected to the effluents treatment process network. in some case, the incineration may not be realized yet, so our process should include a incineration step. | Sewage sludge are obtained daily in high amounts, all over the world, as effluents treatment is obviously a continued process. Indeed this source is '''abondant''' and '''inexhaustible''', as in almost every city around the world, effluents are treated and sewage sludges are collected. In most of the cases, when metals concentrations in sludges are too high to perform a valorization in the environnement as spreading on fields, the procedure is to stock sludges in a confined place. To reduce the stock volume, sludges are burnt.This step is precisely where our process could be connected to the effluents treatment process network. in some case, the incineration may not be realized yet, so our process should include a incineration step. | ||
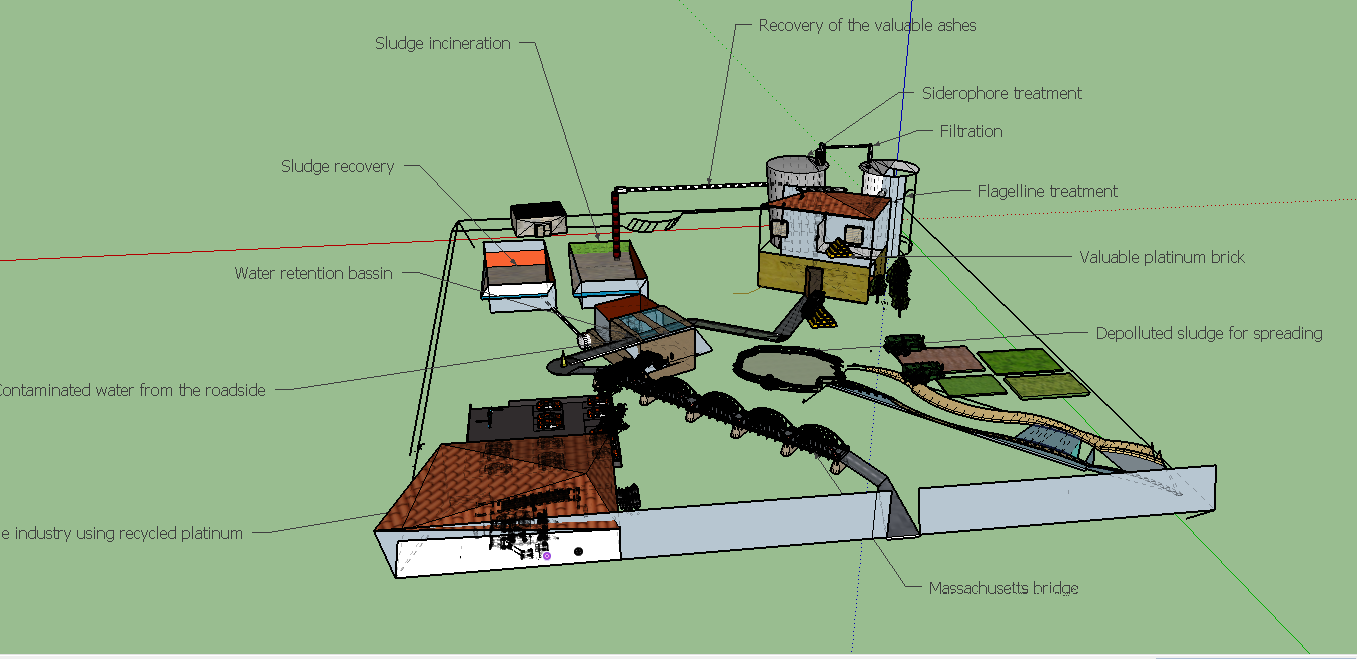
| − | [[File:T--Aix-Marseille--maquette2.jpeg| | + | [[File:T--Aix-Marseille--maquette2.jpeg|800px|center|thumb| Model of facilities of our process. All important elements in the life cycle of platinum, except for the step of production (mines) are displayed here: road, effluents treatment plants, field for spreading of sludge and of course, the facilities where our process would occur]] |
''If we want to harvest '''1g''' of initial platinum, '''1161kg''' to '''3676 kg''' of sewage sludge ashes should be necessary( see [[Team:Aix-Marseille/Integrated_Practices/Process#Required_mass_of_sludge_ashes| Raw Calculations]]). | ''If we want to harvest '''1g''' of initial platinum, '''1161kg''' to '''3676 kg''' of sewage sludge ashes should be necessary( see [[Team:Aix-Marseille/Integrated_Practices/Process#Required_mass_of_sludge_ashes| Raw Calculations]]). | ||
| Line 50: | Line 57: | ||
So where is the innovation in this step? Firstly, in our case, [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] won't be applied on the same materials where is commonly used, in our cases not ores but a leachate of ashes. Basically the main difference will be the metal concentration. | So where is the innovation in this step? Firstly, in our case, [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] won't be applied on the same materials where is commonly used, in our cases not ores but a leachate of ashes. Basically the main difference will be the metal concentration. | ||
| − | Secondly, [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] is usually synthesized chemically, we'll rather produce it in high amounts with bacteria. Indeed, [https://2016.igem.org/Team:Aix-Marseille/Design#Pathway_of_the_desferrioxamine_B_biosynthesis operon] of the Desferrioxamine B biosynthesis from ''Streptomyces coelicolor'' will be cloned into a E. coli bacteria strains in order to produce it, hence lowering the costs of required basic matter as production by | + | Secondly, [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] is usually synthesized chemically, we'll rather produce it in high amounts with bacteria. Indeed, [https://2016.igem.org/Team:Aix-Marseille/Design#Pathway_of_the_desferrioxamine_B_biosynthesis operon] of the Desferrioxamine B biosynthesis from ''Streptomyces coelicolor'' will be cloned into a E. coli bacteria strains in order to produce it, hence lowering the costs of required basic matter as production by bacteria needs especially an appropriate medium and good growth conditions. |
| + | [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] is a derivative of diamines moelcule and therefore its [[Team:Aix-Marseille/Experiments|Biosynthesis]] start with an amino acid, lysine. Lysine is quite expansive, and as we are aware about the cost of our process we decided to use a cheap source of lysine the [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|corn steep liquor]]. Such a lysine source is already in use in industry since it's cheap, amino acid provided, produced in industrial amounts and well known as a excellent source of nitrogen in growth media. So in this step we hope we could produce [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] in high quantities with a affordable cost. Moreover, successful DFHOB production has been reported using corn steep as a source of nitrogen and amino acids<ref> Mehrabi et al., 2010 http://www.ncbi.nlm.nih.gov/pubmed/21313893</ref>. | ||
| + | |||
| + | Following previous uses of [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]], '''78% of platinum''' can be leached with 3mM [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] solution on 100g at 5ppm platinum concentrated ore. That allow us to estimate that in order to reach a 78% yield (max yield obtained) we 'll need to add approximately '''3mg of DFHOB per µg of platinum''' (see [[Team:Aix-Marseille/Integrated_Practices/Process#Quantities_of_DHOB_per_.C2.B5g_of_platinum|Raw calculations]]). Of course this number a based on leaching on ore sample, so maybe we can expect higher yields for the ashes are probably easier to leach than the ore. | ||
| + | |||
| + | |||
| + | ''For a situation of '''1g''' of intial platinum, '''4.68 moles''' of [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] will be needed to leach it (see [[Team:Aix-Marseille/Integrated_Practices/Process#Quantities_of_DHOB_per_.C2.B5g_of_platinum|Raw calculations]]). '' | ||
| + | |||
| + | '''To sum up, this step consist in adding an appropriated quantity of Siderophore producer E. coli to the leachate.''' | ||
| + | |||
| + | ==Siderophore producer lysis== | ||
| + | |||
| + | Our engineered E. coli, i.e. our siderophore producer will produce a lot of [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] molecules in its cytoplasm after induction. But theory tell us that all produced molecules may not go out of the cell hence the membranous export system of E. coli is not adapted to the [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]]. To resolve this we decided to realize the lyse of bacteria in order to release the totality of siderophore molecules in the media. | ||
| + | |||
| + | Several reliable industrial lysis techniques are available to perform this step, essentially centrifugation or filtration. | ||
| + | |||
| + | '''This step consist in lyse the bacterial cells.''' | ||
| + | |||
| + | ==Siderophore recoverer addition== | ||
| + | |||
| + | Until this step, except for the incineration in the first step, actions realized in our process were primarily focused on solubilize the platinum by leaching (acid mediated and siderophore mediated). But if leaching is clearly recommended to improve solubilization of metals, it does not improve the concentrations. | ||
| + | |||
| + | To do so we'll use the ability of ''Streptomyces coelicolor'' to import specifically the [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]]. | ||
| + | Given that the siderophore have been released on the media, the majority of platinum (most of all in a ionic form) is now strongly bounded with it. It has to be noted that [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] specificity is not perfect, so many others metals should from complex with it, especially iron. | ||
| + | In this step we aim to take back the complex siderophore-platinum from the media. As ''Streptomyces coelicolor'' is a [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] producer organism, it possess the appropriate system to import it. That why a certain amount of ''S. coelicolor'' will be added in the growth media during an optimized time of incubation. | ||
| + | |||
| + | We can estimate the amount of bacteria we have to add and also the time of incubation knowing the input capacity of bacteria. | ||
| + | Indeed previous study on uptake of [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] was realized and allow us to estimate time of incubation and amounts of bacteria we have to add in order to recover the maximum of platinum. However the measures of uptake in this study concern [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] bound to iron and not by ''Streptomyces coelicolor'' but by a very close specie, ''Streptomyces pilosus''<ref> Muller and raymond., 1984 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC214717/</ref>. | ||
| + | |||
| + | Following this study, [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] uptake is approximately of '''2.65 nmol/(mg cell(dry weight).h)'''. | ||
| + | |||
| + | It has to be noted that the more cells will be in contact with [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|DFHOB]] the lower will be the cell amount to add (see [[Team:Aix-Marseille/Integrated_Practices/Process#Weight_of_cells_needed_for_importation|Raw calculations]]). The time is the main factor of the total quantity imported: as the metal-siderophore complex is disrupted, the equilibrium of the reaction of importation never will be reached, so bacteria will continue to import siderophore until there is not more left. | ||
| + | |||
| + | ''For a situation with 1 g initial platinum, and with 10 hours of incubation, the wet weight of cells to had will be '''3532 kg''' (see [[Team:Aix-Marseille/Integrated_Practices/Process#Weight_of_cells_needed_for_importation|Raw calculations]]). '' | ||
| + | |||
| + | '''In this step, a certain quantity of ''S. coelicolor'' will be added to the media during determined duration.''' | ||
| + | |||
| + | ==Siderophore recoverer extraction== | ||
| + | |||
| + | At this step, ''S. coelicolor'' cells have imported the vast majority of Siderophore-platinum complexes. The aim of this step is to remove the bacteria from the solution and hence remove platinum with it. Several industrial techniques are relevant to do that as centrifugation or ultrafiltration. At the end of both of these techniques, the cells will be separated from the ashes. | ||
| + | This step allow two important things : | ||
| + | |||
| + | -The sludge ashes contained in the leachate will be rid of the metals especially since the siderophore would have caught a lot of others metals. | ||
| + | This allow the spreading in the fields of the sludge ashes, thus answering the great issue of the sewage sludge problematic. | ||
| + | If the metals concentration are not lowered enough to permit spreading, a additional removing metal process should be performed (step [[Team:Aix-Marseille/Integrated_Practices/Process#Siderophore_mediated_recovery|4]] to [[Team:Aix-Marseille/Integrated_Practices/Process#Siderophore_recoverer_extraction|7]]). | ||
| + | |||
| + | -It move platinum from the whole solution space to the cells cytoplasm space, thus increasing its concentration by reduction of the volume. | ||
| + | |||
| + | The concentration of platinum can be deducted from the weight of cells where it has been imported. So very clearly we can draw a trend: the more [[Team:Aix-Marseille/Integrated_Practices/Process#Siderophore_recoverer_addition| step 6]] is longer, the less cells will be needed to import all siderophore, the more platinum will be concentrated. see | ||
| + | |||
| + | ''In the situation with 1g of initial platinum, with 10 hours of incubation the concentration will be of '''220.84µg/kg''' (see [[Team:Aix-Marseille/Integrated_Practices/Process#Weight_of_cells_needed_for_importation| Raw calculations]]). '' | ||
| + | |||
| + | '''This step consist in removing the cells either by centrifugation or by filtration. At this step, all remained sludge ashes get out of the process.''' | ||
| + | |||
| + | ==Siderophore recoverer destruction== | ||
| + | |||
| + | Once the siderophore has fulfilled its function we need to get rid of it. Indeed the bound between platinum and it is very strong and it would be irrelevant to try to destroy it. That's why we decided to destroy the siderophore itself by a thermal degradation that is to say a incineration. This step will destroy the main componenent of the cells, will remove the water and will destroy most of molecules, including our siderphore. As in the [[Team:Aix-Marseille/Integrated_Practices/Process#The_Source_.28step1.29 |first step]] the burning will reduce the volume of the our materials (from cells to ashes) therefore enhancing its platinum and others metals concentration by '''a X20 factor'''. | ||
| + | |||
| + | ''In the situation with 1 g of initial platinum, the concentration will of '''4.41 mg/kg''' (see [[Team:Aix-Marseille/Integrated_Practices/Process#Concentration_in_the_pellet_ashes| Raw calculations]]). '' | ||
| + | |||
| + | This step present another advantage: in the next step, the most wanted form of platinum is a ionic one and high heat is well known to produce rather ionic particles. Thus the next step will be performed in best conditions. | ||
| + | |||
| + | '''In this step cells filtrates or pellets are incinerated, the rest of the process will be performed with the produced ashes.''' | ||
| + | |||
| + | ==Biosorption== | ||
| + | |||
| + | The aim of its step is the conversion of platinum into its final processed form: nanoparticles. | ||
| + | |||
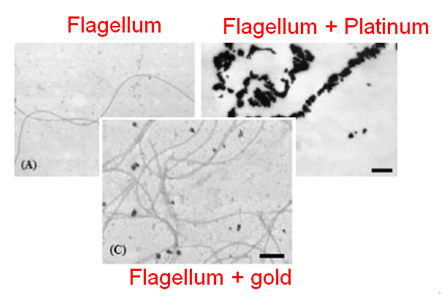
| + | [[Team:Aix-Marseille/Integrated_Practices/Process#Glossary|Biosorption]] can be performed along biological structures since biologic components are known to be excellent sorbents. We planned to perform biosorption along a flagella. Indeed, metallic ions can be sorbed along flagella, see photo.<ref>Deplanche and al., 2008 http://www.ncbi.nlm.nih.gov/pubmed/18819156</ref> thus enhancing concentrations. [[File:Biosfhsdh.png|300px|left|thumb|Adsorption of the platinum on the flagellum, Deplenche & al. 2008]] Moreover this step will form nanoparticles because of the reducing power of biological molecules, especially amines contained in proteins is supposed enough to convert ions into reduced (solid) particles. Optimization experiments will determine if this reducing power is sufficient to perform biosorption. If not, a external reducing power could be brought, i.e. by bubbling gaseous hydrogen in the medium. | ||
| + | |||
| + | Experiments should allow to determine which one of the flagella from either ''Escherichia coli'' or from ''Desulfovibrio desulfuricans'' is the best candidate for biosorption. | ||
| + | In order to optimized the formation of nanoparticles and most of all its specificity on platinum, this step should be realized with engineered flagella by our team, containing small [https://2016.igem.org/Team:Aix-Marseille/Design#Biosorption_and_reduction_using_flagellin_and_peptides platinum catching peptids] <ref>Seker and Demir., 2011 http://eds.a.ebscohost.com.gate1.inist.fr/eds/pdfviewer/pdfviewer?vid=5&sid=77d9b085-94fc-4bd0-8105-229d3ddc0e9c%40sessionmgr4010&hid=4202</ref>. | ||
| + | Given their specificity, it should be enough to ensure the majority of produced nanoparticles will be made up of platinum ones. | ||
| + | |||
| + | In practice, ashes of the previous step will be poured in a engineered purified flagella containing solution and incubate with an optimized temperature an duration. | ||
| + | If we refer to the previous biosorption experiments, the formed nanoparticles should be exhibit a size range from 5nm to 50nm and | ||
| + | once the process is performed, nanoparticles will be display all along flagella, and the levels of ionic platinum particles remained free in the solution should be really low. In fact, in the previous experiments the ratio of platinum mass/flagella mass was 1:1, and the biosorption was complete for all the palladium present in the media (palladium and platinum are very similar elements ans have very similar behaviors). | ||
| + | |||
| + | ''In a siuation with 1g of initial platinum, the ashes will be mixed with '''780 mg''' of purified flagella proteins (see [[Team:Aix-Marseille/Integrated_Practices/Process#Amounts_of_protein_flagella_to_add| Raw calculations]]). '' | ||
| + | |||
| + | '''This step consist mainly in pouring the ashes in a solution containing engineered purified flagella and incubating with an optimized temperature and duration.''' | ||
| + | |||
| + | ==Final recovery== | ||
| + | |||
| + | Now,most of the platinum contained in the solution is now bound to the flagella and in a form of nanoparticles. | ||
| + | |||
| + | The overall complex (nanoparticles+flagella) has a much more higher density than the rest of solution (water and ions), so its isolation will be performed with centrifugation or filtration. As the volume of the fraction of interest should be reduced dramatically (pellet) the concentration in platinum in the isolated fraction should increase in a significant way. However, the more ashes are concentrated the easier will be the separation of the flagella from the rest of solution. If ashes are not concentrated enough, the biosorption won't be performed in good conditions and the volume of ashes should be too important to realize a proper extraction of the flagella. After concentration, the sample is mainly constituted of nanoparticles, hence a very high concentrations. | ||
| + | |||
| + | ''In the situation with 1g of initial platinum, the mass of ashes needed to be centrifuged of filtered will be about '''176 kg'''. If biosorption is realized in optimal conditions, the final concentration in platinum will be about '''500 mg/g''', namely an almost pure solution of solid platinum nanoparticles (see [[Team:Aix-Marseille/Integrated_Practices/Process#Final concentrations|Raw calculations]]''). | ||
| + | |||
| + | '''This final step rely on a simple centrifugation or filtration with the recollection of the fraction containing the flagella. This pellet is highly enriched in platinum nanoparticles and is actually the processed product.''' | ||
| + | |||
| + | ==Further purification steps == | ||
| + | |||
| + | '''This step is not supposed to be tested in lab conditions by our team.''' Besides we can imagine that further purification steps could be wished on the final product in order to a increase the quality of the product. Here a short of steps that could be easily realized with a high efficiency: | ||
| + | |||
| + | -proteolyse | ||
| + | |||
| + | -thermal degradation | ||
| + | |||
| + | -exchanges on an affinity columns.... | ||
| + | |||
| + | ==Prices estimations== | ||
| + | Following the actual uses of platinum nanoparticles, the final purified product presents a high value and is usually sold by specialized companies. | ||
| + | The price is really variable, depending on the purity, the sized of the particles and if the particles have been processed (coating...). | ||
| + | |||
| + | |||
| + | ''In a situation with 1g of initial platinum, with some further purification steps, the final product from our process could have a merchant value ranging from '''150$''' to '''1,560$ ''' even until '''90,000$''' (see [[Team:Aix-Marseille/Integrated_Practices/Process#Prices estimations|Raw calculations]]).'' | ||
| + | |||
| + | ==Glossary== | ||
| + | |||
| + | '''Bioleaching''' : in simple words, leaching is a metal extraction technique which rely on solubilization of metals ores (ore must be soluble and impurities must be insoluble) in a aqueous solution, by using strong acid solutions. The leachate is the solution containing the solubilized metals of interest. Bioleaching in the same process but involves uses of living organism. | ||
| + | |||
| + | '''DFHOB''': Desferioxamine B, is a molecule produced by (among others) ''Streptomyces pilosus'' <ref>Müller, Matzanke and Raymond., 1984 https://www.scopus.com/record/display.uri?eid=2-s2.0-0021137021&origin=inward&txGid=0</ref> able to catch metals (as platinum) with a very high affinity. See the biosynthesis and the cloning process [https://2016.igem.org/Team:Aix-Marseille/Design#Mobilisation_by_a_siderophore here]. | ||
| + | |||
| + | '''Corn steep liquor''': a by-product of an industrial process (wet-milling) applied on corn kernels. As the kernels are steeped in water solution, the process produce an amino acid, vitamins and minerals enriched solution in high volumes. | ||
| + | |||
| + | '''Biosorption''': it is a process which occurs on biomass. It concentrate and bind components onto biological structures by the phenomenon of sorption. The structure is called a sorbent. In industry it can be used to concentrate or to remove an components from a solution. | ||
| + | |||
| + | ==Raw calculations== | ||
| + | |||
| + | ===Required mass of sludge ashes=== | ||
| + | |||
| + | Given that the average platinum concentration<ref>Jackson, Prichard and Sampson., 2009 https://www.ncbi.nlm.nih.gov/pubmed/19878972</ref> in sludges ashes can range from '''272µg/kg''' to '''602 µg/kg''' | ||
| + | So in order to recover 1g of platinum we need a volume estimated to: V=1000/C°*10^-6 | ||
| + | |||
| + | Volume ashes=1000/272*10^-6=3676470g i.e. '''3676kg''' | ||
| + | |||
| + | Volume ashes=1000/602*10^-6=1661129g i.e. '''1661kg''' | ||
| + | |||
| + | ===Quantities of DHOB per µg of platinum=== | ||
| + | |||
| + | Our basic data come from an study realized on the efficency of leaching by DFHOB <ref>Bau and al., 2015 http://dx.doi.org.gate1.inist.fr/10.1016/j.hydromet.2015.01.002</ref>. | ||
| + | |||
| + | An experiment of leaching at pH 8.2 during 120h using DFHOB was carried out on '''100g''' of platinum whose concentration was approximately '''5ppm'''. The maximum efficiency of leaching by siderophore has reached a value of 50% with a DHOB concentration of 3mM (higher concentration lead to a lower efficiency). If we combined the value of leaching occurred with the drop of pH (in the study by Hcl treatment, in our case with ''Thiobacillus'') and the value occurred with leaching, we reach a value of 78% of the total platinum leached. | ||
| + | |||
| + | ==> 100g at 5ppm => 5µg/g, so '''500µg''' of pure platinum. 3mM of DFHOB= 1.68 g given the DFHOB molar mass= 560,684g.mol. So we have roughly (1.68/500) = '''3.36mg of DFHOB/µg of platinum''' We can also have the molar value of '''6 µmol of DFHOB/µg of platinum'''. | ||
| + | |||
| + | ''For a situation of 1g of initial platinum, 78% of the total mean 0.78g. So (6*0.78) = 4.68 moles of DFHOB.'' | ||
| + | |||
| + | ===Weight of cells needed for importation=== | ||
| + | |||
| + | As displayed on the chart, the total mass of cells needed to import all the DFHOB is dependent of the time of incubation. | ||
| + | [[File:T--Aix-Marseille--uptake.png|500px|left]] | ||
| + | |||
| + | |||
| + | ''In a situation with 1 g of initial platinum, during 10h of incubation:'' | ||
| + | |||
| + | ''-the uptake is equal to (2.65*10^(-6)*10)= 2.65*10^(-5) µmol/g of cells (dry wt)'' | ||
| + | |||
| + | ''-the total of DFHOB to import is 4.68 moles so ((4.68/2.65*10^(-5))/1000)= '''176.6 kg''' of dry cells'' | ||
| + | |||
| + | ''- as the dry weight is a tenth of the wet one and that a pellet is composed half by water, the mass of the cell pellet to add will be (176.6*10*2)= '''3532 kg''' '' | ||
| + | |||
| + | ===Concentration in the pellet=== | ||
| + | |||
| + | We consider that all the siderophore bound to the platinum has been imported in the volume of the cells at the end of the incubation. | ||
| + | |||
| + | ''For a situation with 1g of initial platinum,with an incubation of 10 hours, the concentration is (0.78/3532)= '''220.84µg/kg''' | ||
| + | |||
| + | ===Concentration in the pellet ashes=== | ||
| + | |||
| + | [[File:T--Aix-Marseille--uptake2.png|500px|left|Ashes concentrations depends on the incubation]] | ||
| + | Basically, after a combustion a wet cells volume is divided by a factor > 20. | ||
| + | ''In the situation with 1 g initial platinum, with an incubation of 10 hours, the concentration will be (2.20*10^(-4)*20)=''' 4.41 mg/kg'''. | ||
| + | |||
| + | |||
| + | Actually the concentration in the ashes depends primarily on the step of incubation of the [[Team:Aix-Marseille/Integrated_Practices/Process#Siderophore_recoverer_addition|step 6]], as display on the chart. This chart has been realized with predicted values, following a linear increase of the uptake over time. (Values displayed here have not been used for calculation in the others examples.'' | ||
| + | |||
| + | |||
| + | ===Amounts of protein flagella to add=== | ||
| + | The ration of total platinum mass/mass of flagella protein. | ||
| + | |||
| + | ''In a situation with 1 g of initial platinum, the total mass of platinum contained in the ashes is 780 mg, so''' 780 mg''' of protein must be mixed with it.'' | ||
| + | |||
| + | ===Final concentrations=== | ||
| + | If extraction was performed properly, all the rest of the ashes was removed and there is the same mass of platinum and flagella proteins, thus the final concentration. | ||
| + | |||
| + | ''In all cases,'' | ||
| + | ''platinum total mass= Pt'' | ||
| + | |||
| + | ''flagella total mass= Fl'' | ||
| + | |||
| + | ''Final concentration = Pt/(Pt+Fl)'' | ||
| + | ''=Pt/(2Pt)'' | ||
| + | ''=1/2'' | ||
| + | ''='''0.5''' '' | ||
| + | |||
| + | ''In the situation with 1g of initial platinum, the concentration is 4.41 mg/kg and the total mass of platinum is 780 mg so the mass of total ashes is (780/4.41)= '''176 kg''', namely the weigh of dry cells used in the [[Team:Aix-Marseille/Integrated_Practices/Process#Siderophore_recoverer_addition|step 6]].'' | ||
| + | |||
| + | ===Prices estimations=== | ||
| + | |||
| + | Some platinum prices estimations have been made relying in available prices in websites. Let's consider the 780 mg produced in the final step are available at the end of the further purification steps : | ||
| + | |||
| + | -200 nm sized nanparticles ([http://ssnano.com/inc/sdetail/platinum_nanoparticles/8217?gclid=Cj0KEQjwyJi_BRDLusby7_S7z-IBEiQAwCVvn4XH1pLOVQgRyj_fi_2SmvWo0k2nTc8JxmrJ_aoEWu8aArZ58P8HAQ| Seller]): '''192$/g''': in our case 780mg worth '''150$''' | ||
| + | |||
| + | -10 nm sized nanoparticles ([http://www.sciventions.com/product_info.php?cPath=19_44&products_id=126&gclid=Cj0KEQjwyJi_BRDLusby7_S7z-IBEiQAwCVvn0uxkOwlip3945inSj1ZLbi2VU4ySIg4oG_pNODOMGgaAvqa8P8HAQ| Seller]) : '''2000$/g''' in our case, 780 mg worth '''1560$''' | ||
| + | |||
| + | -50 nm sized nanoparticle purified coated with sodium citrate surface ([http://nanocomposix.eu/collections/platinum-nanoparticles/products/50-nm-platinum-nanoparticles| Seller]): roughly '''114,833$/g''': in our case 780mg worth approximately '''90,000$'''. | ||
| + | |||
| + | |||
| + | <references/> | ||
| + | |||
| + | {{:Team:Aix-Marseille/Template-Footer}} | ||
Contents
- 1 Our Process is applicable on many ways
- 2 The Source
- 3 Bioleaching
- 4 Siderophore mediated leaching
- 5 Siderophore producer lysis
- 6 Siderophore recoverer addition
- 7 Siderophore recoverer extraction
- 8 Siderophore recoverer destruction
- 9 Biosorption
- 10 Final recovery
- 11 Further purification steps
- 12 Prices estimations
- 13 Glossary
- 14 Raw calculations
Our process
- Metals importance throughout history
- Platinum in mines
- Platinum in industry
- Platinum in the environment
- Our process
In this page we developed all estimation and reflexion we made around our process. Thanks to Pr. Sigoillot from the INRA (The French National Institute of Agronomy) we have been able to carry out a relevant process engineering study around application our process could permit.
For the detailed interview with Pr.Sigoillot see here.
Our Process is applicable on many ways
Our process concentrate metals, especially platinum and transform it into a highly valuable form, nanoparticles.
To obtain this valuable product, our process begin with raw, worthless, and plentiful materials that can be found almost anywhere since the accumulation due to the catalyst converter occurs everywhere there is a car traffic (urban and road areas). The substrates can be harvest directly in the environment, as some of them presents impressive concentrations like the road dust or the air borne dust. However,it would be much more relevant to integrate our process at the end of an already existing process of treatment, to plug it to the actual network of process. Moreover, solutions of phytoremediation provide a lot of substrates and by-products our project could start from. As most of our process occurs in a controlled environment almost any substrate could be convenient to enter in our process. We could start our process just after the incineration of phytoremedial plants or on the digestat produced by a methanisation
If the raw materials change (substrate), only the first step of our project would change if the basic matter change. The advantage of our process is its easiness of integration into current treatment process. Set up our process would not need a lot of changes in actual facilities.
In this part we ll'detail an industrialized process of what we plan to test in lab condition. We are thankfull to Mr.Sigoillot, who advised us wisely in the choice of which process could be more developed in particular, see the interview here. We'll focus on a process started from sewage sludge. Indeed, choosing sewage sludge as a source of platinum bring another benefit: it will remove platinum from sludges. Nowadays, sludge are so concentrated in metals that it can't be spread on field for valorisation and as a result, sludge are often burnt and ashes are kept in specialized confinement centers, thus making the treatment very expensive and not sustainable. So our process can remove metals aiming to recover it and doing so our process will also achieve the purpose to rid metals from sludges. Moreover, sewage sludge treatment is a payed service by institutions who needs to get rid of it (municipality, motorway operating...) so with this source of platinum our process will start with an already positive financial balance! Of courses polluting metals, preventing spreading in field are many and our process is currently designed to recover specifically and only platinum. But our process is extremely versatile and each step of our process can be modified to be specific of another metal (see video below).
As you will discover in the process, step 4 involves a siderophore that could be specific to another metal. Likewise, the biosorption occurred in the step 9 involves small metals catching peptids, that can be used to catch far more other metals that just only platinum.
During all the explanation of the process, examples displayed in italic are considered for the recovery of 1g of pure platinum.
The Source
Sewage sludge are obtained daily in high amounts, all over the world, as effluents treatment is obviously a continued process. Indeed this source is abondant and inexhaustible, as in almost every city around the world, effluents are treated and sewage sludges are collected. In most of the cases, when metals concentrations in sludges are too high to perform a valorization in the environnement as spreading on fields, the procedure is to stock sludges in a confined place. To reduce the stock volume, sludges are burnt.This step is precisely where our process could be connected to the effluents treatment process network. in some case, the incineration may not be realized yet, so our process should include a incineration step.
If we want to harvest 1g of initial platinum, 1161kg to 3676 kg of sewage sludge ashes should be necessary( see Raw Calculations).
In this first step sludges are simply collected from the effluents treatment network and eventually incinerated thus the product here is ashes.
Bioleaching
The Bioleaching process allow a far better recovery of platinum. Indeed, the drop of pH is required for the metals solubilization. This step could be realized with chemicals as chlorhydric acid in the actual industry. But in order to achieve a greener process as possible, we decided to lower the pH with biological ways, as the leaching accomplished by Thiobacillus. This method is widely use in mines, e.g. for copper mines in South America, so this will be a reliable step already tested in industrial conditions. This method rely on the ability of the bacteria Thiobacilus to acidify its medium until a pH of 1. The bacterium solution is applied on the ashes and liquid part dropping from it constitute the leachate i.e. a very acidified solutions containing solubilized metals particles mostly in ionic form. With this process we hope reach the same leach yield as obtained if realized with Chlorhydric acid i.e. 30% of leached platinum[1].
This step wouldn't be obligatory in our process, but it could improve our recovery yield while still using a environmentally friendly approach. Moreover, after leaching, the remained ashes will present a decreased concentration in metals. If this concentrations are lowered enough, the ashes are now ready to be spreaded on fields. Of courses before it, steps of alcanization and destruction of potentially Thiobacillus cells should be performed. If concentrations are still too high for spreading, this step may be repeated.
This step consist in spreading a Thiobacillus solution on the ashes then recollecting the produced leachate. This step could be also realised not with Thiobacillus but with chemicals solutions.
Siderophore mediated leaching
Even if almost 30 % of platinum has been leached by the drop of pH we need to improve the proportion of leached platinum. As we worked with synthetic biology, we decided to use what it is commonly employed by cells to catch metals, i.e. siderophore. Siderophores are well known to catch iron most of all but some of them have an affinity with others metals as platinum. So in our process, we planned to work with such a siderophore, called Desferrioxamine B. This one is already employed to leach platinum in mines, and have shown high capacity to leach platinum from ores [2].
Leaching yields with DFHOB can reach 78% of the total platinum if both of these conditions are fully respected: a lowering pH level leaching step should be performed, as well as a alcanization of medium (until a pH range between 8 and 9) just before addition of siderophore. The last step devoted to rise up the pH level will be realized using a standard buffer, e.g. a Tris buffer.
So where is the innovation in this step? Firstly, in our case, DFHOB won't be applied on the same materials where is commonly used, in our cases not ores but a leachate of ashes. Basically the main difference will be the metal concentration. Secondly, DFHOB is usually synthesized chemically, we'll rather produce it in high amounts with bacteria. Indeed, operon of the Desferrioxamine B biosynthesis from Streptomyces coelicolor will be cloned into a E. coli bacteria strains in order to produce it, hence lowering the costs of required basic matter as production by bacteria needs especially an appropriate medium and good growth conditions. DFHOB is a derivative of diamines moelcule and therefore its Biosynthesis start with an amino acid, lysine. Lysine is quite expansive, and as we are aware about the cost of our process we decided to use a cheap source of lysine the corn steep liquor. Such a lysine source is already in use in industry since it's cheap, amino acid provided, produced in industrial amounts and well known as a excellent source of nitrogen in growth media. So in this step we hope we could produce DFHOB in high quantities with a affordable cost. Moreover, successful DFHOB production has been reported using corn steep as a source of nitrogen and amino acids[3].
Following previous uses of DFHOB, 78% of platinum can be leached with 3mM DFHOB solution on 100g at 5ppm platinum concentrated ore. That allow us to estimate that in order to reach a 78% yield (max yield obtained) we 'll need to add approximately 3mg of DFHOB per µg of platinum (see Raw calculations). Of course this number a based on leaching on ore sample, so maybe we can expect higher yields for the ashes are probably easier to leach than the ore.
For a situation of 1g of intial platinum, 4.68 moles of DFHOB will be needed to leach it (see Raw calculations).
To sum up, this step consist in adding an appropriated quantity of Siderophore producer E. coli to the leachate.
Siderophore producer lysis
Our engineered E. coli, i.e. our siderophore producer will produce a lot of DFHOB molecules in its cytoplasm after induction. But theory tell us that all produced molecules may not go out of the cell hence the membranous export system of E. coli is not adapted to the DFHOB. To resolve this we decided to realize the lyse of bacteria in order to release the totality of siderophore molecules in the media.
Several reliable industrial lysis techniques are available to perform this step, essentially centrifugation or filtration.
This step consist in lyse the bacterial cells.
Siderophore recoverer addition
Until this step, except for the incineration in the first step, actions realized in our process were primarily focused on solubilize the platinum by leaching (acid mediated and siderophore mediated). But if leaching is clearly recommended to improve solubilization of metals, it does not improve the concentrations.
To do so we'll use the ability of Streptomyces coelicolor to import specifically the DFHOB. Given that the siderophore have been released on the media, the majority of platinum (most of all in a ionic form) is now strongly bounded with it. It has to be noted that DFHOB specificity is not perfect, so many others metals should from complex with it, especially iron. In this step we aim to take back the complex siderophore-platinum from the media. As Streptomyces coelicolor is a DFHOB producer organism, it possess the appropriate system to import it. That why a certain amount of S. coelicolor will be added in the growth media during an optimized time of incubation.
We can estimate the amount of bacteria we have to add and also the time of incubation knowing the input capacity of bacteria. Indeed previous study on uptake of DFHOB was realized and allow us to estimate time of incubation and amounts of bacteria we have to add in order to recover the maximum of platinum. However the measures of uptake in this study concern DFHOB bound to iron and not by Streptomyces coelicolor but by a very close specie, Streptomyces pilosus[4].
Following this study, DFHOB uptake is approximately of 2.65 nmol/(mg cell(dry weight).h).
It has to be noted that the more cells will be in contact with DFHOB the lower will be the cell amount to add (see Raw calculations). The time is the main factor of the total quantity imported: as the metal-siderophore complex is disrupted, the equilibrium of the reaction of importation never will be reached, so bacteria will continue to import siderophore until there is not more left.
For a situation with 1 g initial platinum, and with 10 hours of incubation, the wet weight of cells to had will be 3532 kg (see Raw calculations).
In this step, a certain quantity of S. coelicolor will be added to the media during determined duration.
Siderophore recoverer extraction
At this step, S. coelicolor cells have imported the vast majority of Siderophore-platinum complexes. The aim of this step is to remove the bacteria from the solution and hence remove platinum with it. Several industrial techniques are relevant to do that as centrifugation or ultrafiltration. At the end of both of these techniques, the cells will be separated from the ashes. This step allow two important things :
-The sludge ashes contained in the leachate will be rid of the metals especially since the siderophore would have caught a lot of others metals. This allow the spreading in the fields of the sludge ashes, thus answering the great issue of the sewage sludge problematic. If the metals concentration are not lowered enough to permit spreading, a additional removing metal process should be performed (step 4 to 7).
-It move platinum from the whole solution space to the cells cytoplasm space, thus increasing its concentration by reduction of the volume.
The concentration of platinum can be deducted from the weight of cells where it has been imported. So very clearly we can draw a trend: the more step 6 is longer, the less cells will be needed to import all siderophore, the more platinum will be concentrated. see
In the situation with 1g of initial platinum, with 10 hours of incubation the concentration will be of 220.84µg/kg (see Raw calculations).
This step consist in removing the cells either by centrifugation or by filtration. At this step, all remained sludge ashes get out of the process.
Siderophore recoverer destruction
Once the siderophore has fulfilled its function we need to get rid of it. Indeed the bound between platinum and it is very strong and it would be irrelevant to try to destroy it. That's why we decided to destroy the siderophore itself by a thermal degradation that is to say a incineration. This step will destroy the main componenent of the cells, will remove the water and will destroy most of molecules, including our siderphore. As in the first step the burning will reduce the volume of the our materials (from cells to ashes) therefore enhancing its platinum and others metals concentration by a X20 factor.
In the situation with 1 g of initial platinum, the concentration will of 4.41 mg/kg (see Raw calculations).
This step present another advantage: in the next step, the most wanted form of platinum is a ionic one and high heat is well known to produce rather ionic particles. Thus the next step will be performed in best conditions.
In this step cells filtrates or pellets are incinerated, the rest of the process will be performed with the produced ashes.
Biosorption
The aim of its step is the conversion of platinum into its final processed form: nanoparticles.
Biosorption can be performed along biological structures since biologic components are known to be excellent sorbents. We planned to perform biosorption along a flagella. Indeed, metallic ions can be sorbed along flagella, see photo.[5] thus enhancing concentrations. Moreover this step will form nanoparticles because of the reducing power of biological molecules, especially amines contained in proteins is supposed enough to convert ions into reduced (solid) particles. Optimization experiments will determine if this reducing power is sufficient to perform biosorption. If not, a external reducing power could be brought, i.e. by bubbling gaseous hydrogen in the medium.Experiments should allow to determine which one of the flagella from either Escherichia coli or from Desulfovibrio desulfuricans is the best candidate for biosorption. In order to optimized the formation of nanoparticles and most of all its specificity on platinum, this step should be realized with engineered flagella by our team, containing small platinum catching peptids [6]. Given their specificity, it should be enough to ensure the majority of produced nanoparticles will be made up of platinum ones.
In practice, ashes of the previous step will be poured in a engineered purified flagella containing solution and incubate with an optimized temperature an duration. If we refer to the previous biosorption experiments, the formed nanoparticles should be exhibit a size range from 5nm to 50nm and once the process is performed, nanoparticles will be display all along flagella, and the levels of ionic platinum particles remained free in the solution should be really low. In fact, in the previous experiments the ratio of platinum mass/flagella mass was 1:1, and the biosorption was complete for all the palladium present in the media (palladium and platinum are very similar elements ans have very similar behaviors).
In a siuation with 1g of initial platinum, the ashes will be mixed with 780 mg of purified flagella proteins (see Raw calculations).
This step consist mainly in pouring the ashes in a solution containing engineered purified flagella and incubating with an optimized temperature and duration.
Final recovery
Now,most of the platinum contained in the solution is now bound to the flagella and in a form of nanoparticles.
The overall complex (nanoparticles+flagella) has a much more higher density than the rest of solution (water and ions), so its isolation will be performed with centrifugation or filtration. As the volume of the fraction of interest should be reduced dramatically (pellet) the concentration in platinum in the isolated fraction should increase in a significant way. However, the more ashes are concentrated the easier will be the separation of the flagella from the rest of solution. If ashes are not concentrated enough, the biosorption won't be performed in good conditions and the volume of ashes should be too important to realize a proper extraction of the flagella. After concentration, the sample is mainly constituted of nanoparticles, hence a very high concentrations.
In the situation with 1g of initial platinum, the mass of ashes needed to be centrifuged of filtered will be about 176 kg. If biosorption is realized in optimal conditions, the final concentration in platinum will be about 500 mg/g, namely an almost pure solution of solid platinum nanoparticles (see Raw calculations).
This final step rely on a simple centrifugation or filtration with the recollection of the fraction containing the flagella. This pellet is highly enriched in platinum nanoparticles and is actually the processed product.
Further purification steps
This step is not supposed to be tested in lab conditions by our team. Besides we can imagine that further purification steps could be wished on the final product in order to a increase the quality of the product. Here a short of steps that could be easily realized with a high efficiency:
-proteolyse
-thermal degradation
-exchanges on an affinity columns....
Prices estimations
Following the actual uses of platinum nanoparticles, the final purified product presents a high value and is usually sold by specialized companies. The price is really variable, depending on the purity, the sized of the particles and if the particles have been processed (coating...).
In a situation with 1g of initial platinum, with some further purification steps, the final product from our process could have a merchant value ranging from 150$ to 1,560$ even until 90,000$ (see Raw calculations).
Glossary
Bioleaching : in simple words, leaching is a metal extraction technique which rely on solubilization of metals ores (ore must be soluble and impurities must be insoluble) in a aqueous solution, by using strong acid solutions. The leachate is the solution containing the solubilized metals of interest. Bioleaching in the same process but involves uses of living organism.
DFHOB: Desferioxamine B, is a molecule produced by (among others) Streptomyces pilosus [7] able to catch metals (as platinum) with a very high affinity. See the biosynthesis and the cloning process here.
Corn steep liquor: a by-product of an industrial process (wet-milling) applied on corn kernels. As the kernels are steeped in water solution, the process produce an amino acid, vitamins and minerals enriched solution in high volumes.
Biosorption: it is a process which occurs on biomass. It concentrate and bind components onto biological structures by the phenomenon of sorption. The structure is called a sorbent. In industry it can be used to concentrate or to remove an components from a solution.
Raw calculations
Required mass of sludge ashes
Given that the average platinum concentration[8] in sludges ashes can range from 272µg/kg to 602 µg/kg So in order to recover 1g of platinum we need a volume estimated to: V=1000/C°*10^-6
Volume ashes=1000/272*10^-6=3676470g i.e. 3676kg
Volume ashes=1000/602*10^-6=1661129g i.e. 1661kg
Quantities of DHOB per µg of platinum
Our basic data come from an study realized on the efficency of leaching by DFHOB [9].
An experiment of leaching at pH 8.2 during 120h using DFHOB was carried out on 100g of platinum whose concentration was approximately 5ppm. The maximum efficiency of leaching by siderophore has reached a value of 50% with a DHOB concentration of 3mM (higher concentration lead to a lower efficiency). If we combined the value of leaching occurred with the drop of pH (in the study by Hcl treatment, in our case with Thiobacillus) and the value occurred with leaching, we reach a value of 78% of the total platinum leached.
==> 100g at 5ppm => 5µg/g, so 500µg of pure platinum. 3mM of DFHOB= 1.68 g given the DFHOB molar mass= 560,684g.mol. So we have roughly (1.68/500) = 3.36mg of DFHOB/µg of platinum We can also have the molar value of 6 µmol of DFHOB/µg of platinum.
For a situation of 1g of initial platinum, 78% of the total mean 0.78g. So (6*0.78) = 4.68 moles of DFHOB.
Weight of cells needed for importation
As displayed on the chart, the total mass of cells needed to import all the DFHOB is dependent of the time of incubation.
In a situation with 1 g of initial platinum, during 10h of incubation:
-the uptake is equal to (2.65*10^(-6)*10)= 2.65*10^(-5) µmol/g of cells (dry wt)
-the total of DFHOB to import is 4.68 moles so ((4.68/2.65*10^(-5))/1000)= 176.6 kg of dry cells
- as the dry weight is a tenth of the wet one and that a pellet is composed half by water, the mass of the cell pellet to add will be (176.6*10*2)= 3532 kg
Concentration in the pellet
We consider that all the siderophore bound to the platinum has been imported in the volume of the cells at the end of the incubation.
For a situation with 1g of initial platinum,with an incubation of 10 hours, the concentration is (0.78/3532)= 220.84µg/kg
Concentration in the pellet ashes
Basically, after a combustion a wet cells volume is divided by a factor > 20. In the situation with 1 g initial platinum, with an incubation of 10 hours, the concentration will be (2.20*10^(-4)*20)= 4.41 mg/kg.
Actually the concentration in the ashes depends primarily on the step of incubation of the step 6, as display on the chart. This chart has been realized with predicted values, following a linear increase of the uptake over time. (Values displayed here have not been used for calculation in the others examples.
Amounts of protein flagella to add
The ration of total platinum mass/mass of flagella protein.
In a situation with 1 g of initial platinum, the total mass of platinum contained in the ashes is 780 mg, so 780 mg of protein must be mixed with it.
Final concentrations
If extraction was performed properly, all the rest of the ashes was removed and there is the same mass of platinum and flagella proteins, thus the final concentration.
In all cases, platinum total mass= Pt
flagella total mass= Fl
Final concentration = Pt/(Pt+Fl) =Pt/(2Pt) =1/2 =0.5
In the situation with 1g of initial platinum, the concentration is 4.41 mg/kg and the total mass of platinum is 780 mg so the mass of total ashes is (780/4.41)= 176 kg, namely the weigh of dry cells used in the step 6.
Prices estimations
Some platinum prices estimations have been made relying in available prices in websites. Let's consider the 780 mg produced in the final step are available at the end of the further purification steps :
-200 nm sized nanparticles ([http://ssnano.com/inc/sdetail/platinum_nanoparticles/8217?gclid=Cj0KEQjwyJi_BRDLusby7_S7z-IBEiQAwCVvn4XH1pLOVQgRyj_fi_2SmvWo0k2nTc8JxmrJ_aoEWu8aArZ58P8HAQ| Seller]): 192$/g: in our case 780mg worth 150$
-10 nm sized nanoparticles ([http://www.sciventions.com/product_info.php?cPath=19_44&products_id=126&gclid=Cj0KEQjwyJi_BRDLusby7_S7z-IBEiQAwCVvn0uxkOwlip3945inSj1ZLbi2VU4ySIg4oG_pNODOMGgaAvqa8P8HAQ| Seller]) : 2000$/g in our case, 780 mg worth 1560$
-50 nm sized nanoparticle purified coated with sodium citrate surface ([http://nanocomposix.eu/collections/platinum-nanoparticles/products/50-nm-platinum-nanoparticles| Seller]): roughly 114,833$/g: in our case 780mg worth approximately 90,000$.
- ↑ Bau and al., 2015 http://dx.doi.org.gate1.inist.fr/10.1016/j.hydromet.2015.01.002
- ↑ Bau and al., 2015 http://dx.doi.org.gate1.inist.fr/10.1016/j.hydromet.2015.01.002
- ↑ Mehrabi et al., 2010 http://www.ncbi.nlm.nih.gov/pubmed/21313893
- ↑ Muller and raymond., 1984 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC214717/
- ↑ Deplanche and al., 2008 http://www.ncbi.nlm.nih.gov/pubmed/18819156
- ↑ Seker and Demir., 2011 http://eds.a.ebscohost.com.gate1.inist.fr/eds/pdfviewer/pdfviewer?vid=5&sid=77d9b085-94fc-4bd0-8105-229d3ddc0e9c%40sessionmgr4010&hid=4202
- ↑ Müller, Matzanke and Raymond., 1984 https://www.scopus.com/record/display.uri?eid=2-s2.0-0021137021&origin=inward&txGid=0
- ↑ Jackson, Prichard and Sampson., 2009 https://www.ncbi.nlm.nih.gov/pubmed/19878972
- ↑ Bau and al., 2015 http://dx.doi.org.gate1.inist.fr/10.1016/j.hydromet.2015.01.002