Crepuscule (Talk | contribs) |
Crepuscule (Talk | contribs) |
||
| Line 2: | Line 2: | ||
{{:Team:Tianjin/Templates/AddCSS|:Team:Tianjin/Experiment/R-R/style.css}} | {{:Team:Tianjin/Templates/AddCSS|:Team:Tianjin/Experiment/R-R/style.css}} | ||
| + | {{:Team:Tianjin/Templates/AddCSS|:Team:Tianjin/Community/css/bootstrap.css}} | ||
<html> | <html> | ||
<head> | <head> | ||
| Line 486: | Line 487: | ||
</p> | </p> | ||
<div align="center"> <img src="https://static.igem.org/mediawiki/2016/9/9e/T--Tianjin--experiment-11.jpg" alt="desktop"></div><br/> | <div align="center"> <img src="https://static.igem.org/mediawiki/2016/9/9e/T--Tianjin--experiment-11.jpg" alt="desktop"></div><br/> | ||
| − | <p style="font-size:18px">Culture them at 30℃ & 200 rpm, checked the bacterial concentration at OD<sub>600</sub> and detect the concentration of TPA by UV at OD<sub>242</sub>, and then observe | + | <p style="font-size:18px">Culture them at 30℃ & 200 rpm, checked the bacterial concentration at OD<sub>600</sub> and detect the concentration of TPA by UV at OD<sub>242</sub>, and then observe s |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
Revision as of 13:13, 18 October 2016
Bacteria Consortium
Overview
After Yoshida and his co-workers found and isolated Ideonella sakaiensis 201-F6, which produced two enzymes to degrades PET, we kept very high interests at their works and also came up with many ordinary ideas to increase the efficiency of degradation reaction. Bacteria consortium is one of the most creative ideas.
The inspiration of this idea comes from nature and also learns from nature. Actually, bacteria never exist alone in our nature, they co-work and cooperate together to achieve an aim or live better in a special condition. Thinking from this point, we established a special bacteria consortium for this enzyme catalysis reaction.
1. Optimization of Culture Conditions
In order to improve efficiency of degrading PET, we are determined to co-culture Pseudomonas putida KT2440, Rhodococcus jostii RHA1 and Bacillus stubtilis 168 (or Bacillus stubtilis DB 104). In our bacteria consortium, work of degradation is divided several parts as follows:
1.Rhodococcus jostii RHA1 is responsible for degrading TPA (terephthalic acid) to remove substrate inhibition;
2. Pseudomonas putida KT2440 is responsible for degrading EG (ethylene glycol) to remove substrate inhibition, and contribute to produce degradable plastics PHA (polyhydroxyalkanoate).
3. Bacillus stubtilis 168 (or Bacillus stubtilis DB 104) is responsible for secreting PETase and MHETase as the main player of degrading PET.
Whereas, if our bacteria consortium want to achieve their aim, they must work in harmony, therefore, it is necessary find a appropriate environment where these bacteria can normally or supernormally work together.
Primarily, we try several kinds of medium and decide to use W medium in the end; next, we optimize culture conditions by change carbon source, nitrogen source and some ions, then, we check growing situations and conditions of the degrading PET, TPA and EG; eventually, 1we can find out a suitable culture condition to co-culture our bacteria consortium.
2. Modification of Pseudomonas putida KT2440
P.putida KT2440 is one of bacteria which can utilize ethylene glycol (EG) at a high speed and meanwhile produces mcl-PHA. In 1988, Lageveen and his co-workers first found mcl-PHA in P.putida KT2440. And then, the metabolism of producing PHA in P.putida KT2440 was reasearched, which found the gene AcoA was the key gene in the procedure. José Manuel Borrero-de Acuña and his co-workers improved the yield by 33% by overexpressing AcoA.
From Björn Mückschel’s works, Ethylene Glycol Metabolism by Pseudomonas putida was found. The key enzymes were identified by comparative proteomics. In P. putida JM37, tartronate semialdehyde synthase (Gcl), malate synthase (GlcB), and isocitrate lyase (AceA) were found to be induced in the presence of ethylene glycol or glyoxylic acid. Under the same conditions, strain KT2440 showed induction of AceA only.
From those studies, we decided to overexpress AcoA and AceA in P.putida KT2440 to help utilize EG as energy source for its growth.
3. Modification of Bacillus subtilis
After some attempt in E.coli and yeast, we look for a new type of host cells- B.subtilis for more secretion. In our experiment, the genes encoding two enzymes are for the first time expressed in S.cerevisiae. Increased yields of PETase and MHETase enzymes are achieved when B. subtilis strains 168 and DB104 (deficient in two and three extracellular proteases, respectively[1]) were transformed with the recombinant plasmid with the help of the enhanced promoter-p43.
4. A Controllable Lipid Producer
Cyanobacteria are excellent organisms for biofuel production. We thus have selected Cyanobacterium Synechocystis sp. PCC 6803 as the source of carbon in our mixed bacteria system. Our target is simply to make the cyanobacteria lyse at the appropriate time by transforming a plasmid contained three bacteriophage-derived lysis genes which were placed downstream of a nickel-inducible signal transduction system into the Synechocystis 6803.
In this part of our project, Cyanobacterium Synechocystis sp. PCC 6803 was selected as a model organism as the source of carbon in our mixed bacteria system. We simply to establish a cell wall disruption process which could make the cyanobacteria lyse at the appropriate time.
Theoretical Background
1. Degradation of Terephthalate
Rhodococcus sp. strain RHA1 is thought to be capable of degrading a wide range of aromatic compounds including terephthalate acid (TPA). in 2006, a reliable pathway consisting of Distinct ring cleavage dioxygenase systems and protocatechuate (PCA) pathway was come up with, and the proposed degradation pathway for TPA is shown as below[2].

2. Degradation of Ethylene Glycol
By employing growth and bioconversion experiments, directed mutagenesis, and proteome analysis, it is found that Pseudomonas putida KT2440 does not grow within 2 days of incubation, compared to Pseudomonas putida JM37 which can grow rapidly under the same conditions. The key enzymes and specific differences between the two strains were identified by comparative proteomics. In P. putida JM37, tartronate semialdehyde synthase (Gcl), malate synthase (GlcB), and isocitrate lyase (AceA) were found to be induced in the presence of ethylene glycol or glyoxylic acid. Under the same conditions, strain KT2440 showed induction of AceA only. Postulated pathway for the metabolism of ethylene glycol in Pseudomonas putida strains KT2440 and JM37 is shown left[3].
4. Advantages of B. subtilis strains
Bacillus subtilis has an excellent secretion ability, displays fast growth, and is a nonpathogenic bacterium free of endotoxin [5] . It can, therefore, be used in food, enzyme, and pharmaceutical industries and can replace Escherichia coli for protein expression. Furthermore, the extracellular heterogeneous proteins secreted from B. subtilis are more convenient for recovery and purification in large-scale production during downstream processing [6] .
5. Enhanced Promoter-p43
In order to increase secretion, some enhanced promoters are necessary. However, native gene in a high-copy number plasmid was found to be unstable in B. subtilis[5] . To optimize the production and the stability of the expression vectors, both the promoter and the signal sequence of PETase were replaced by B. subtilis P43 promoter, a constitutively expressed promoter. This overcame the plasmid instability problem.
6. The Cooperation of Two Promoters-p43+psacB
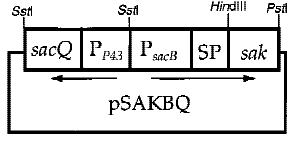
Interestingly, the cooperation of two promoters in B. subtilis are easily found. For example, An endoglucanase from Bacillus akibai I-1 was successfully overexpressed in Bacillus subtilis 168 by the help of p43 promoter and the expression level of the recombinant enzyme was greatly enhanced by using the sucrose-inducible psacB promoter[7] .The construction of plasmid is in the Figure 1 . Thus, we are willing to try whether the combination of p43 and psacB can make a difference in the secretion of enzyme.
7. Lipid Producer
Photosynthetic microorganisms, including eukaryotic algae and cyanobacteria, are being optimized to overproduce numerous biofuel. According to previous data, algae accumulate large quantities of lipid as storage materials, but they do this when under stress and growing slowly. By contrast, cyanobacteria accumulate lipids in thylakoid membranes, which are associated with high levels of photosynthesis and a rapid growth rate. Thus, photo-synthetic bacteria have a natural advantage for producing lipids at a high rate. Furthermore, being prokaryotes can be improved by genetic manipulations much more readily than can eukaryotic algae. [8] Therefore, we decided to do something to make cyanobacteria ,the lipid producer, more appropriate for our project.
Synechococcus elongatus PCC7942 has larger capacity of lipid production than Synechocystis sp. PCC6803 but accumulates most of the product in the cell because of the imbalance of the rates of lipid production and secretion. Initially, we intended to do something to increase lipid secretion by knocking the wzt gene[9] (Akihiro Kato et al. 2016), however, Synechococcus elongatus PCC7942 wasn’t able to revive in two-week shaking cultivation. So we turned into Synechocystis sp.PCC 6803.
8. Lipid Recovery From Biomass
The first goal of our research was to facilitate lipid recovery from biomass. The scientific community widespread disrupts the cyanobacterial cell envelope to achieve the goal. [10] (Seog JL et al. 1998)However, all these methods are not economical for large amounts of biomass or add additional cost and reduce the overall utility of the process. Our target is simply to make the cyanobacteria lyse at the appropriate time. We found that the cyanobacterial cell envelope is composed of 4 layers: the external surface layers ;the outer membrane; the polypeptidoglycan which is considerably thick, and the cytoplasmic membrane. [11] ( Hoiczyk E et al. 2000)To break up the peptidoglycan layer, we applied the holin-endolysin lysis strategy used by bacteriophages to exit bacterial cells[12] (Wang IN et al. 2000). Endolysins are peptidoglycan-degrading enzymes that attack the covalent linkages of the peptidoglycans that maintain the integrity of the cell wall. In addition to endolysins, some auxiliary lysis factors are involved in cleaving the oligopeptide linkages between the peptidoglycan and the outer membrane lipoprotein. Holins are small membrane proteins that produce nonspecific lesions (holes) in the
cytoplasmic membrane from within, allow the endolysins and auxiliary lysis factors to gain access to the polypeptidoglycan layers, and trigger the lysis process. In this way, the cell wall is easy to break up.
9. Control The Lysis System
To control the appropriate time, a nickel sensing/responding signal system[13] (Garcia-Dominguez M et al. 2000) was used to control the timing of the expression of phage lysis genes in Synechocystis 6803.
Our strategy for achieving our target is to construct a expression vector pCPC3031-Ni-13-19-15 introduced the Salmonella phage P22 lysis cassette (13-19-15) with a Spectinomycin selection marker downstream of the promoter Pni, a nickel responding signal operon. Synechocystis 6803 with the pCPC3031-Ni-13-19-15 will lyse after Ni2+ addition.
Experiment Design
Optimization of Culture Conditions
1. Find an Appropriate Medium
Co-culture different pairs in improved W medium[17] in the same condition (using two tubes in each group):

Co-culture different pairs in improved M9 medium in the same condition (using two tubes in each group):

Co-culture different pairs in LB medium in the same condition(using two tubes in each group):

Co-culture different pairs in YPD medium in the same condition (using two tubes in each group):

(PS: OD600 of all the above bacteria solutions are 0.60, and these bacteria solutions are got by diluting all kinds of bacteria cultured in LB or YPD medium; and similarly hereinafter.)
Culture all groups at 30℃ & 200 rpm for 3 days, check the bacterial concentration at OD600 and observe these groups with microscope.
(P.S. We always extract 200μL samples to each well in 96-well plate, and similarly hereinafter.)
Eventually, we find W medium is most promising to co-culture these bacteria.
2. Nitrogen Source
We added some kinds of nitrogenous organic and inorganic compounds to W medium to improve initial W medium, and strategies of improvement are shown as follows.

Add bacteria solution to media above as following table (use two tubes each group)

Culture them at 30℃ & 200 rpm, checked the bacterial concentration at OD600 and detect the concentration of TPA by UV at OD242, and then observe some samples with microscope.
3. Carbon Source
We added some different kinds of carbonic organic compounds to W medium to improve initial W medium, and strategies of improvement are shown as follows.

Add bacteria solution to media above as following table (use two tubes each group)

Culture them at 30℃ & 200 rpm, checked the bacterial concentration at OD600 and detect the concentration of TPA by UV at OD242, and then observe some samples with microscope.
4. Mg2+ and Cl-
We add Mg2+ and Cl- to improve initial W medium, and strategies of improvement is shown as follows.

Add bacteria solution to media above as following table (use two tubes each group)

Culture them at 30℃ & 200 rpm, checked the bacterial concentration at OD600 and detect the concentration of TPA by UV at OD242, and then observe some samples with microscope.
5. Orthogonal Experiments
We added some different kinds of nitrogenous organic or inorganic compounds and carbonic organic compounds to W medium to improve initial W medium, and strategies of improvement are shown as follows.

(PS: Because concentration of TPA is lower actually, we decrease concentration of sodium terephthalate.)
Add bacteria solution to mediums above as following table (use two tubes each group)

Culture them at 30℃ & 200 rpm, checked the bacterial concentration at OD600 and detect the concentration of TPA by UV at OD242, and then observe some samples with microscope.
6. Temperture
Culture bacteria as following strategies at 37℃ & 200 rpm

Checked the bacterial concentration at OD600.
7. Preliminary Experiments for Degrading PET
We prepared media as following strategies:

(PS: Because concentration of TPA is lower actually, we decrease concentration of sodium terephthalate.)
Add bacteria solution to mediums above as following table (use two tubes each group)

Culture them at 30℃ & 200 rpm, checked the bacterial concentration at OD600 and detect the concentration of TPA by UV at OD242, and then observe s