Zigapusnik (Talk | contribs) |
Zigapusnik (Talk | contribs) (Undo revision 306862 by Zigapusnik (talk)) |
||
| Line 2: | Line 2: | ||
<html> | <html> | ||
<head> | <head> | ||
| − | <title> | + | <title>Gas vesicles</title> |
<link rel="stylesheet" | <link rel="stylesheet" | ||
href="//2016.igem.org/Team:Slovenia/libraries/semantic-min-css?action=raw&ctype=text/css"> | href="//2016.igem.org/Team:Slovenia/libraries/semantic-min-css?action=raw&ctype=text/css"> | ||
| Line 73: | Line 73: | ||
<!-- menu goes here --> | <!-- menu goes here --> | ||
<!-- content goes here --> | <!-- content goes here --> | ||
| − | + | <div class="main ui citing justified container"> | |
| − | + | <h1 class = "ui left dividing header"><span class="section"> </span>Enhanced Mechanosensitivity by Gas Vesicles expressed in Mammalian Cells</h1> | |
| + | |||
| + | |||
<div class = "ui segment" style = "background-color: #ebc7c7; "> | <div class = "ui segment" style = "background-color: #ebc7c7; "> | ||
| − | <p><b><ul><li> | + | <p><b><ul><li>Addition of synthetic lipid microbubbles improved the responsiveness of cells to low-power ultrasound. |
| − | <li> | + | <li>Gas vesicle-forming proteins were expressed in mammalian cells where they improved sensitivity of cells to ultrasound. |
| − | + | <li>Combination of the ectopic expression of the mechanosensing bacterial channel MscS and gas vesicle-forming proteins sensitized cells to mechanical stimulation.</ul></b> | |
| − | </div> | + | </p> |
| − | + | </div> | |
| + | |||
| + | |||
<div class = "ui segment"> | <div class = "ui segment"> | ||
| − | <p> | + | <h3><span id = "motivation" class="section"> </span></h3> |
| − | + | <p>For activation of mechanoreceptors TRPC1 or MscS, a high-power ultrasound wave (900 Vpp) is required. Our aim was to improve responsiveness of cells to respond to the lower power of ultrasound as this would increase the selectivity, avoiding stimulation of endogenous channels and prevent cell damage. We decided to test gas-filled lipid microbubbles, since it has been reported that microbubbles can amplify the ultrasonic signal <x-ref> Ibsen2015 </x-ref>.</p> | |
| − | + | ||
| − | + | ||
<div class="ui styled fluid accordion"> | <div class="ui styled fluid accordion"> | ||
<div class="title"> | <div class="title"> | ||
| Line 93: | Line 95: | ||
</div> | </div> | ||
<div class="content"> | <div class="content"> | ||
| − | <p> | + | <p> |
| − | + | Microbubbles are small gas-filled lipid vesicles which are used as contrast agents in medicine. Their size is in the range of micrometers. They work by resonating in an ultrasound beam, rapidly contracting and expanding in response to the pressure changes of the sound wave<x-ref>Blomley2001</x-ref>. | |
| − | + | Ibsen et al. <x-ref>Ibsen2015</x-ref> have used microbubbles for transduction of the ultrasonic wave in order to make neurons of <i>C.elegans</i> sensitive to ultrasound. | |
| − | + | ||
</p> | </p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</div> | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| − | + | ||
| − | + | <div> | |
| − | <h1><span class="section"> </span>Results</h1> | + | <h1><span id = "results" class="section"> </span>Results</h1> |
| − | <div class = "ui segment"> | + | <div class = "ui segment"> |
| − | <h4><span class="section"> </span> | + | <h4><span id = "results" class="section"> </span>Microbubbles</h4> |
| − | + | <div style = "float:right;"> | |
| − | + | <figure data-ref="3.3.1."> | |
| − | + | <img class="ui large image" src="https://static.igem.org/mediawiki/2016/4/48/T--Slovenia--3.3.1.PNG"> | |
| − | + | <figcaption><b>Synthetic lipid microbubbles.</b><br>(A)Schematic of a cell with an increased sensitivity to ultrasound stimulation in the presence of microbubbles. When exposed to mechanical stimuli the microbubbles contract and expand, resulting in activation of mechanosensitive channels on the cell membrane. (B) Mixed size distribution of lipid microbubbles was obtained. Stabilized lipid microbubbles were prepared by sonication and detected by microscopy. | |
| − | <div style = " | + | </figcaption> |
| − | <figure data-ref="3 | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | <img class="ui | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</figure> | </figure> | ||
</div> | </div> | ||
| − | + | <p>Properties of microbubbles for example rigidity, are affected by the composition of the lipid membrane and the gas core. We prepared our lipid microbubbles from a mixture of DSPC:DSPE. Before sonication we added gas perfluorohexane (as described in the Protocols section), which facilitates compression and expansion of the microbubbles upon ultrasound stimulation (<ref>3.3.1.</ref>A). A heterogeneous mixture of microbubbles in the range from 5 to 100 <span>µ</span>m in size were generated by this procedure (<ref>3.3.1.</ref>B). Microbubbles are most effective in the size range corresponding to the resonance frequency of the ultrasound. However care has to be taken in the applied energy to prevent cavitation, that can sonoporate cell membranes.</p> | |
| − | < | + | <p style="clear:both">Application of microbubbles to cells expressing mechanosensitive channel MscS significantly improved calcium influx after mechanical stimulation using low-power ultrasound wave (450 Vpp) (<ref>3.3.2.</ref>).</p> |
| − | <figure data-ref=" | + | <div style = "clear:left;" align ="center"> |
| − | <img class="ui big centered image" src=" | + | <figure data-ref="3.3.2."> |
| − | + | <img class="ui big centered image" src="//2016.igem.org/wiki/images/7/7e/T--Slovenia--3.3.2.png" > | |
| − | + | <!-- Žiga, vstavi GIF--> | |
| − | + | <div style = "clear:both; display:block; width: 90%; margin-right: auto; margin-left: auto"> | |
| − | + | <div style = "display: block; float: left; width: 80%;"> | |
| + | <!-- experiment: 20160729 MscS + MB --> | ||
| + | <img class = "playme" id = "gifGroup10" src = "//2016.igem.org/wiki/images/5/59/T--Slovenia--Group101.png" width = "100%" data-alt="//2016.igem.org/wiki/images/9/9c/T--Slovenia--20160729_masina_3_MscS_10ul_100_redcenih_MB_graf.gif"> | ||
| + | </div> | ||
| + | <div> | ||
| + | <!-- experiment: 20160729 MscS + MB --> | ||
| + | <div style = "display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup10" src="//2016.igem.org/wiki/images/0/01/T--Slovenia--Group102.png", width = "100%" data-alt="//2016.igem.org/wiki/images/d/db/T--Slovenia--20160729_masina_3_MscS_10ul_100_redcenih_MB_regions.gif"> | ||
</div> | </div> | ||
| − | + | <div style = "display: block; float: left; width: 20%;"> | |
| − | + | <img class="gifGroup10" src="//2016.igem.org/wiki/images/d/de/T--Slovenia--Group103.png", width = "100%" data-alt="//2016.igem.org/wiki/images/7/72/T--Slovenia--20160729_masina_3_MscS_10ul_100_redcenih_MB_controls.gif"> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</div> | </div> | ||
| − | </div> | + | <div style = "display: block; float: left; width: 20%;"> |
| − | <figcaption><b> | + | <img class="gifGroup10" src="//2016.igem.org/wiki/images/6/63/T--Slovenia--Group104.png", width = "100%" data-alt="//2016.igem.org/wiki/images/d/d9/T--Slovenia--20160729_masina_3_MscS_10ul_100_redcenih_MB_heatmap.gif"> |
| − | + | </div> | |
| − | (C | + | </div> |
| − | + | </div> | |
| − | + | ||
| − | + | ||
| − | + | <figcaption><b> Ultrasound response of cells is amplified by the use of lipid microbubbles. </b><br/>(A) Presentation of the ultrasound stimulation sequence and (B) signal parameters used for the stimulation. | |
| + | (C, D) Lipid microbubbles strongly enhanced response of cells expressing MscS channels. HEK293 cells expressing MscS channels were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. For comparison cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE. | ||
| + | </figcaption> | ||
</figure> | </figure> | ||
| + | </div> | ||
| + | <p>However, there are some drawbacks related to the use of lipid microbubbles, as their delivery requires injection into the selected tissue. Additionally lifetime of lipid microbubbles is limited in the tissue to tens of minutes and they need to be prepared freshly at least once a week.</p> | ||
| + | <p>To overcome the described drawback we thought of alternative options. One idea that initially looked too crazy to work was to use genetically encoded gas vesicles that are produced in bacteria. Bacterial gas vesicles have been used as contrasting agents for ultrasonography in animals <x-ref> Shapiro2014</x-ref>. This demonstrated that ultrasound can have effect on gas vesicles. However adding bacterial gas vesicles instead of microbubbles would not solve the problem. The best solution would be if the gas vesicles could be produced in the functional form in mammalian cells. iGEM team OUC_China 2012 demonstrated that only two protein components were sufficient to prepare functional gas vesicles in <i>E.coli</i>. Since gas vesicles are compressible their size should vary with pressure variations in the ultrasound or by mechanical stimulus, which would strongly influence the cytoskeleton or cell membrane therefore they would likely amplify activation of mechano-channels.</p> | ||
| + | <div style = "display:block; float:left; width:55%"> | ||
| + | <p> | ||
| + | <br /> | ||
| + | </p> | ||
| + | <div class="ui styled fluid accordion" style="clear:both;"> | ||
| + | <div class="title"> | ||
| + | <i class="dropdown icon"></i> | ||
| + | Further explanation ... | ||
| + | </div> | ||
| + | <div class="content"> | ||
| + | <p> | ||
| + | Gas vesicles are stable gas-filled structures, which provide buoyancy in a wide variety of planktonic prokaryotes <x-ref> Buchholz1993</x-ref> Two structural proteins have been identified in the gas vesicles of cyanobacteria and the halophilic archaea. The major protein, GvpA, is small hydrophobic protein that forms the ribs of the gas vesicle wall, while the minor gas vesicle protein, GvpC, is larger, hydrophilic protein that stabilizes the structure<x-ref>Hayes1986</x-ref>. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | <p> | ||
| + | <br /> | ||
| + | </p> | ||
</div> | </div> | ||
| − | < | + | <div style="float:right;width:45%;"> |
| − | + | <figure data-ref="3.3.3."> | |
| − | + | <img src="//2016.igem.org/wiki/images/a/a4/T--Slovenia--3.3.3.png" > | |
| − | + | <figcaption><b>Expression of gas vesicle forming proteins in mammalian cells. </b><br/> Expression of (A) GvpC and (B) GvpA protein was determined by Western blot using (A) anti-FLAG and (B) anti-AU1, respectively. Expected sizes (marked with arrow) are 22,5 kDa and 8,5 kDa for GvpC and GvpA, respectively. | |
| − | <div style = " | + | </figcaption> |
| − | <figure data-ref=" | + | </figure> |
| − | <img | + | </div> |
| − | + | <p>HEK293 cells were transfected with plasmids expressing both gas vesicle forming proteins, GvpA and GvpC. We could obtain GvpC from the Registry and added to its characterization, while the plasmid for GvpA could not be recovered and its coding sequence was synthesized using mammalian codon usage. Expression of both proteins was confirmed by the western blot (<ref>3.3.3.</ref>) and colocalization was observed by confocal microscopy (<ref>3.3.4.</ref>).</p> | |
| − | + | <p style = "clear:both;"></p> | |
| + | <div style = "float:left; width:70%"> | ||
| + | <figure data-ref="3.3.4."> | ||
| + | <img src="//2016.igem.org/wiki/images/f/fc/T--Slovenia--3.3.4.png"> | ||
| + | <figcaption><b> GvpA and GvpC were both expressed in the cytosol and colocalized.</b><br/><p style="text-align:justify;"> HEK293T cells were transfected with plasmids encoding gas vesicle proteins, GvpA and GvpC. 24 h after transfection cells were fixed, permeabilized and immunostained with anti-FLAG (upper row) and anti-AU1 (lower row). Both GvpA and GvpC are located in cytosol as expected. Colocalization is presented in the overlay picture.</p></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <div style="float:left; width:30%;"> | ||
| + | <figure data-ref="3.3.5."> | ||
| + | <img src="//2016.igem.org/wiki/images/6/62/T--Slovenia--3.3.5.png"> | ||
| + | <figcaption><b> Cell viability was not affected by expression of gas vesicle forming proteins.</b><br/> HEK293 cells were transfected with GvpA and/or GvpC. 24 h after the transfection viability of cells was measured using trypan blue. | ||
| + | </figcaption> | ||
| + | </figure> | ||
</div> | </div> | ||
| − | + | <p>A toxicity test was performed in order to ensure that gas vesicles were not toxic to mammalian cells. <ref>3.3.5.</ref> shows that the viability of cells was not altered when expressing gas vesicle forming proteins.</p> | |
| − | <div style = "clear:both;" align = "center"> | + | <p style = "clear:both;">HEK293 cells expressing gas vesicle-forming proteins exhibited increased sensitivity to ultrasound stimulation, even in the absence of exogenous mechanosensitive channels (<ref>3.3.6.</ref>), which was most likely due to activation of the endogenous mechanosensitive channels in mammalian cells.</p> |
| − | <figure data-ref=" | + | <div style = "clear:both;" align="center"> |
| − | <img class="ui big centered image" src="https://static.igem.org/mediawiki/2016/ | + | <figure data-ref="3.3.6."> |
| − | + | <img class="ui big centered image" src="https://static.igem.org/mediawiki/2016/a/aa/T--Slovenia--3.3.6.png"> | |
| − | + | <div style = "clear:both; display:block; width: 90%; margin-right: auto; margin-left: auto"> | |
| − | + | <div style = "display: block; float: left; width: 80%;"> | |
| − | + | <!--experiment: 20160816 1 pcDNA + Gvp 200V 118s --> | |
| − | + | <img class = "playme" id = "gifGroup1" src = "//2016.igem.org/wiki/images/e/eb/T--Slovenia--Group11.png" width = "100%" data-alt="//2016.igem.org/wiki/images/2/2c/T--Slovenia--20160816_1_pcDNA_Gvp_200V_118s_graf.gif"> | |
| + | </div> | ||
| + | <div> | ||
| + | <!--experiment: 20160816 1 pcDNA + Gvp 200V 118s --> | ||
| + | <div style = "display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup1" src="//2016.igem.org/wiki/images/4/4b/T--Slovenia--Grou13.png", width = "100%" data-alt="//2016.igem.org/wiki/images/4/4b/T--Slovenia--20160816_1_pcDNA_Gvp_200V_118s_region.gif"> | ||
</div> | </div> | ||
| − | + | <div style = "display: block; float: left; width: 20%;"> | |
| − | + | <img class="gifGroup1" src="//2016.igem.org/wiki/images/b/b6/T--Slovenia--Group14.png", width = "100%" data-alt="//2016.igem.org/wiki/images/8/87/T--Slovenia--20160816_1_pcDNA_Gvp_200V_118s_control.gif"> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</div> | </div> | ||
| − | + | <div style = "display: block; float: left; width: 20%;"> | |
| − | <figcaption><b> | + | <img class="gifGroup1" src="//2016.igem.org/wiki/images/2/2d/T--Slovenia--Group12.png", width = "100%" data-alt="//2016.igem.org/wiki/images/e/ec/T--Slovenia--20160816_1_pcDNA_Gvp_200V_118s_heatmap.gif"> |
| − | + | </div> | |
| − | + | </div> | |
| − | + | </div> | |
| − | + | <figcaption><b> Gas protein vesicles improve the sensitivity of cells to ultrasound.</b><br/>(A) Presentation of the ultrasound stimulation sequences for 900 Vpp (red) and 450 Vpp (grey) Vpp ultrasound waves and (B) signal parameters used for stimulation. (C, D) Genetically encoded gas vesicle-forming proteins greatly increased cell response at high-power (900 Vpp) in comparison to low-power ultrasound (450 Vpp). HEK293 cells expressing Gvps were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D). Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE. | |
| − | + | </figcaption> | |
</figure> | </figure> | ||
| − | </div> | + | </div> |
| − | <p | + | <p>In order to maximize the sensitivity of cells to ultrasound, we transfected cells with a combination of mechanosensitive channel MscS and gas vesicle-forming proteins. By decreasing the power of ultrasound stimulation we demonstrated that cells expressing a combination of both ectopic channels and gas vesicles were activated as a result of the ultrasound stimulation (<ref>3.3.7.</ref>).</p> |
| − | + | <div style="clear:both;" align="center"> | |
| − | + | <figure data-ref="3.3.7."> | |
| − | </p> | + | <img class="ui big centered image" src="https://static.igem.org/mediawiki/2016/9/99/T--Slovenia--3.3.7.png"> |
| − | + | <div style = "clear:both; display:block; width: 90%; margin-right: auto; margin-left: auto"> | |
| − | + | <div style = "display: block; float: left; width: 80%;"> | |
| − | + | <!-- experiment: 20160729 MscS + MB --> | |
| − | + | <img class = "playme" id = "gifGroup10" src = "//2016.igem.org/wiki/images/9/98/T--Slovenia--Group41.png" width = "100%" data-alt="//2016.igem.org/wiki/images/2/2a/T--Slovenia--20160819_5_MscS_Gvp_50Vgraf.gif"> | |
| − | + | </div> | |
| − | + | <div> | |
| − | + | <!-- experiment: 20160729 MscS + MB --> | |
| − | + | <div style = "display: block; float: left; width: 20%;"> | |
| + | <img class="gifGroup10" src="//2016.igem.org/wiki/images/8/83/T--Slovenia--Group42.png", width = "100%" data-alt="//2016.igem.org/wiki/images/b/b4/T--Slovenia--20160819_5_MscS_Gvp_50Vregion.gif"> | ||
| + | </div> | ||
| + | <div style = "display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup10" src="//2016.igem.org/wiki/images/7/7a/T--Slovenia--Group43.png", width = "100%" data-alt="//2016.igem.org/wiki/images/0/0e/T--Slovenia--20160819_5_MscS_Gvp_50Vcontrol.gif"> | ||
| + | </div> | ||
| + | <div style = "display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup10" src="//2016.igem.org/wiki/images/a/a8/T--Slovenia--Group44.png", width = "100%" data-alt="//2016.igem.org/wiki/images/2/2b/T--Slovenia--20160819_5_MscS_Gvp_50Vheatmap.gif"> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <figcaption><b> Coexpression MscS with Gvps in mammalian cells improves sensitivity of cells to ultrasound even at lower voltages.</b><br/>(A) Presentation of the ultrasound stimulation sequence and (B) signal parameters used for stimulation. | ||
| + | (C, D) Co-expression of mechanosensitive channels and gas vesicle-forming proteins increased sensitivity to ultrasound stimulation in comparison to the cells without exogenous mechanosensitive channels. | ||
| + | HEK293 cells expressing gas vesicle-forming proteins GvpA and GvpC with or without MscS were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. Changes in fluorescence intensity of calcium indicators Fluo-4 (green line) and Fura Red (red line) are shown. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.</figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <p style="clear:both;">To prove that calcium influx was the mediator of activation of mechanosensitive channels, we used an inhibitor of ion channels gadolinium (Gd3+), which has high charge density and similar ionic radius to Ca2+ .By blocking the pore of the channel it acts as an general inhibitor of calcium ion channels <x-ref>Bourne1982</x-ref> . As shown on <ref>3.3.8.</ref>, the addition of gadolinium prevented calcium influx triggered by ultrasound stimulation.</p> | ||
| + | <div style="clear:both;" align = "center"> | ||
| + | <figure data-ref="3.3.8."> | ||
| + | <img class="ui big centered image" src="https://static.igem.org/mediawiki/2016/4/4c/T--Slovenia--3.3.8.png"> | ||
| + | <div style = "clear:both; display:block; width: 90%; margin-right: auto; margin-left: auto"> | ||
| + | <div style = "display: block; float: left; width: 80%;"> | ||
| + | <!-- experiment: 20161002 2 MscS + Gvp 200V 90s --> | ||
| + | <img class = "playme" id = "gifGroup8" src = "//2016.igem.org/wiki/images/e/e1/T--Slovenia--Group81.png" width = "100%" data-alt="//2016.igem.org/wiki/images/3/3b/T--Slovenia--20161002_2_MscS_%2B_Gvp_200V_90s_graf.gif"> | ||
| + | </div> | ||
| + | <div> | ||
| + | <!-- experiment: 20161002 2 MscS + Gvp 200V 90s --> | ||
| + | <div style = "display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup8" src="//2016.igem.org/wiki/images/c/c0/T--Slovenia--Group82.png", width = "100%" data-alt="//2016.igem.org/wiki/images/e/e2/T--Slovenia--20161002_2_MscS_%2B_Gvp_200V_90s_Regions.gif"> | ||
| + | </div> | ||
| + | <div style = "display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup8" src="//2016.igem.org/wiki/images/9/97/T--Slovenia--Group83.png", width = "100%" data-alt="//2016.igem.org/wiki/images/7/72/T--Slovenia--20161002_2_MscS_%2B_Gvp_200V_90s_Controls.gif"> | ||
| + | </div> | ||
| + | <div style = "display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup8" src="//2016.igem.org/wiki/images/3/31/T--Slovenia--Group84.png", width = "100%" data-alt="//2016.igem.org/wiki/images/f/ff/T--Slovenia--20161002_2_MscS_2B_Gvp_200V_90s_HeatMap.gif"> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <figcaption><b> Calcium influx after ultrasound stimulation is mediated by activation of mechanosensitive channels.</b><br/>(A) Presentation of the ultrasound stimulation sequence and (B) signal parameters used for stimulation. (C, D) Gadolinium inhibits activation of mechanosensitive ion channels after ultrasound stimulation. HEK293 cells expressing gas vesicle-forming proteins GvpA and GvpC with or without MscS were treated with gadolinium (red line) or not (grey line) and stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D). Changes in fluorescence intensity of calcium indicators Fluo-4 (green line) and Fura Red (red line) are shown. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.</figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | <p><br /><br />This is the first time demonstration that that gas vesicle-forming proteins can be expressed in human cells and that they improve the sensitivity of mechanosensitive channels for ultrasound. Moreover when the gas vesicle-forming proteins were co-expressed with ectopic ion channels the calcium influx could be achieved with low-power ultrasound.</p> | ||
| + | <p>Ultrasound is a type of mechanical stimulus, therefore we reasoned that cells might exhibit response also to other types of stimulus such as the touch. The next task was to couple the response of mechanosensitive channels to the mediator of signaling or to the genetically encoded reporter. Mediator of this activation are Ca2+ ions, which are sensed by different cellular proteins and which have been in the literature detected by several designed sensors. The mechanosensing device was then coupled to the calcium split luciferase reporter based on <a href="//2016.igem.org/Team:Slovenia/Mechanosensing/CaDependent_mediator">M13 and calmodulin</a> or can be coupled to the <a href="//2016.igem.org/Team:Slovenia/Protease_signaling/Split_proteases">protease split system</a> and response of cells was determined against mechanical stimulus in a <a href="//2016.igem.org/Team:Slovenia/Implementation/Touch_painting">Touchpaint implementation</a>.</p> | ||
| + | <p style="clear:both;"></p> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 311: | Line 278: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | |||
<script> | <script> | ||
Revision as of 16:28, 17 October 2016
Enhanced Mechanosensitivity by Gas Vesicles expressed in Mammalian Cells
For activation of mechanoreceptors TRPC1 or MscS, a high-power ultrasound wave (900 Vpp) is required. Our aim was to improve responsiveness of cells to respond to the lower power of ultrasound as this would increase the selectivity, avoiding stimulation of endogenous channels and prevent cell damage. We decided to test gas-filled lipid microbubbles, since it has been reported that microbubbles can amplify the ultrasonic signal
Microbubbles are small gas-filled lipid vesicles which are used as contrast agents in medicine. Their size is in the range of micrometers. They work by resonating in an ultrasound beam, rapidly contracting and expanding in response to the pressure changes of the sound wave
Results
Microbubbles

(A)Schematic of a cell with an increased sensitivity to ultrasound stimulation in the presence of microbubbles. When exposed to mechanical stimuli the microbubbles contract and expand, resulting in activation of mechanosensitive channels on the cell membrane. (B) Mixed size distribution of lipid microbubbles was obtained. Stabilized lipid microbubbles were prepared by sonication and detected by microscopy.
Properties of microbubbles for example rigidity, are affected by the composition of the lipid membrane and the gas core. We prepared our lipid microbubbles from a mixture of DSPC:DSPE. Before sonication we added gas perfluorohexane (as described in the Protocols section), which facilitates compression and expansion of the microbubbles upon ultrasound stimulation (3.3.1.A). A heterogeneous mixture of microbubbles in the range from 5 to 100 µm in size were generated by this procedure (3.3.1.B). Microbubbles are most effective in the size range corresponding to the resonance frequency of the ultrasound. However care has to be taken in the applied energy to prevent cavitation, that can sonoporate cell membranes.
Application of microbubbles to cells expressing mechanosensitive channel MscS significantly improved calcium influx after mechanical stimulation using low-power ultrasound wave (450 Vpp) (3.3.2.).
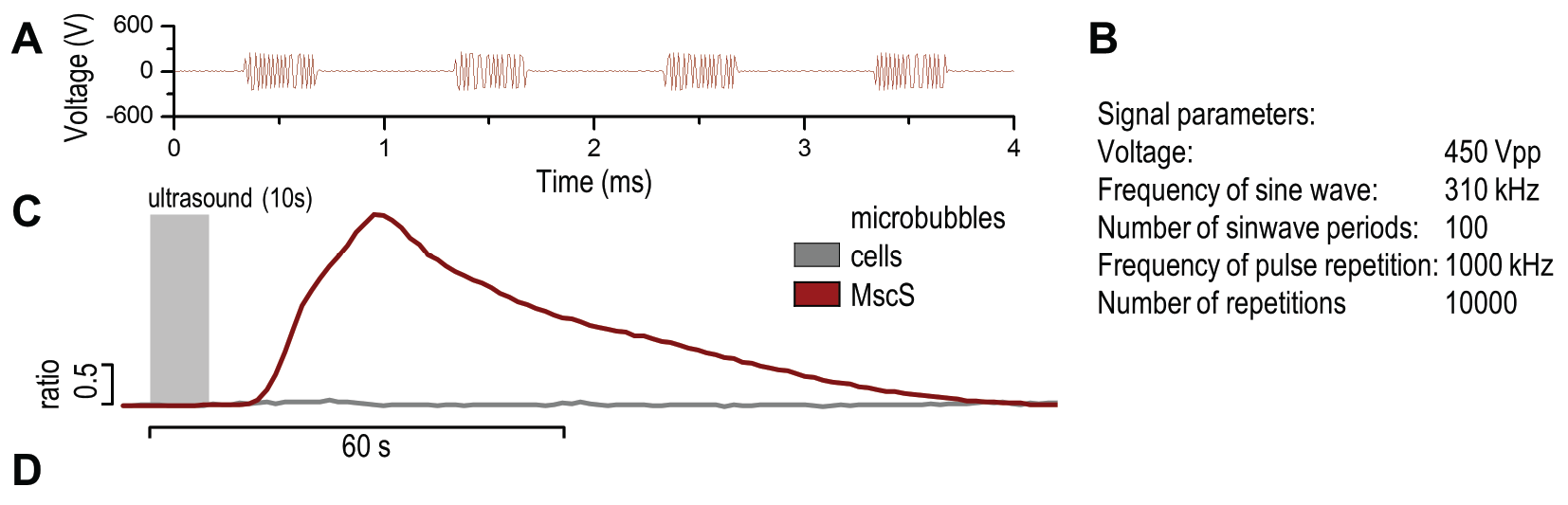
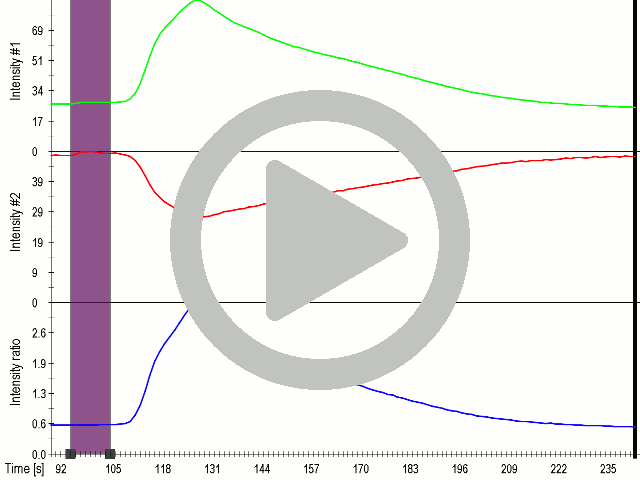

(A) Presentation of the ultrasound stimulation sequence and (B) signal parameters used for the stimulation. (C, D) Lipid microbubbles strongly enhanced response of cells expressing MscS channels. HEK293 cells expressing MscS channels were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. For comparison cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
However, there are some drawbacks related to the use of lipid microbubbles, as their delivery requires injection into the selected tissue. Additionally lifetime of lipid microbubbles is limited in the tissue to tens of minutes and they need to be prepared freshly at least once a week.
To overcome the described drawback we thought of alternative options. One idea that initially looked too crazy to work was to use genetically encoded gas vesicles that are produced in bacteria. Bacterial gas vesicles have been used as contrasting agents for ultrasonography in animals
Gas vesicles are stable gas-filled structures, which provide buoyancy in a wide variety of planktonic prokaryotes
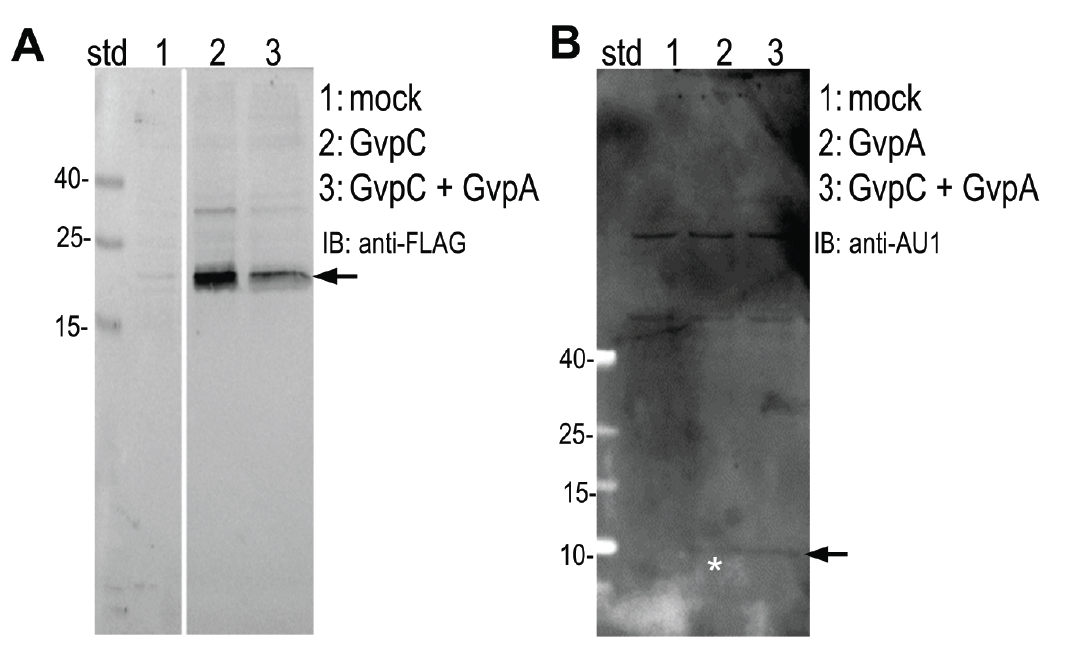
Expression of (A) GvpC and (B) GvpA protein was determined by Western blot using (A) anti-FLAG and (B) anti-AU1, respectively. Expected sizes (marked with arrow) are 22,5 kDa and 8,5 kDa for GvpC and GvpA, respectively.
HEK293 cells were transfected with plasmids expressing both gas vesicle forming proteins, GvpA and GvpC. We could obtain GvpC from the Registry and added to its characterization, while the plasmid for GvpA could not be recovered and its coding sequence was synthesized using mammalian codon usage. Expression of both proteins was confirmed by the western blot (3.3.3.) and colocalization was observed by confocal microscopy (3.3.4.).
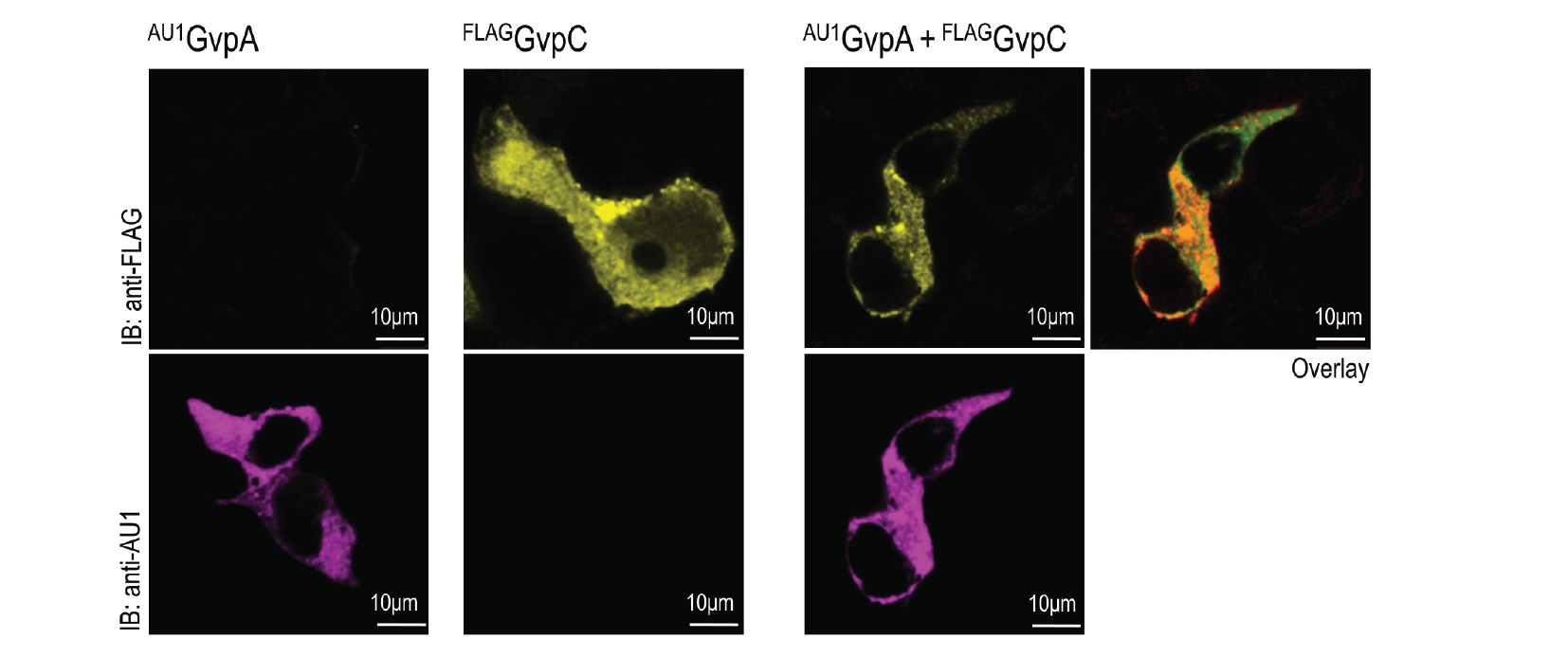
HEK293T cells were transfected with plasmids encoding gas vesicle proteins, GvpA and GvpC. 24 h after transfection cells were fixed, permeabilized and immunostained with anti-FLAG (upper row) and anti-AU1 (lower row). Both GvpA and GvpC are located in cytosol as expected. Colocalization is presented in the overlay picture.
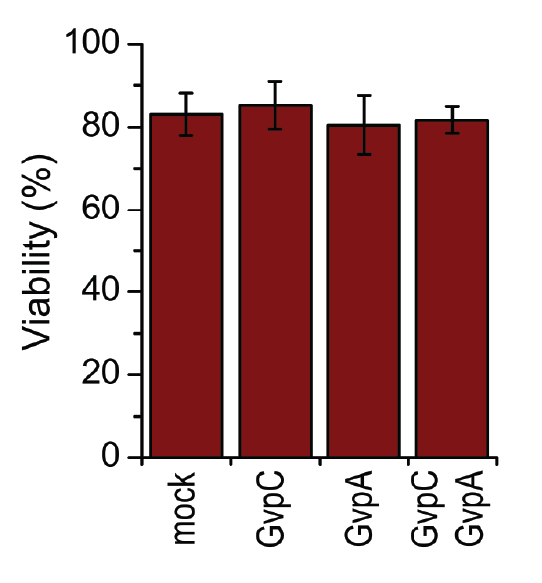
HEK293 cells were transfected with GvpA and/or GvpC. 24 h after the transfection viability of cells was measured using trypan blue.
A toxicity test was performed in order to ensure that gas vesicles were not toxic to mammalian cells. 3.3.5. shows that the viability of cells was not altered when expressing gas vesicle forming proteins.
HEK293 cells expressing gas vesicle-forming proteins exhibited increased sensitivity to ultrasound stimulation, even in the absence of exogenous mechanosensitive channels (3.3.6.), which was most likely due to activation of the endogenous mechanosensitive channels in mammalian cells.



(A) Presentation of the ultrasound stimulation sequences for 900 Vpp (red) and 450 Vpp (grey) Vpp ultrasound waves and (B) signal parameters used for stimulation. (C, D) Genetically encoded gas vesicle-forming proteins greatly increased cell response at high-power (900 Vpp) in comparison to low-power ultrasound (450 Vpp). HEK293 cells expressing Gvps were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D). Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
In order to maximize the sensitivity of cells to ultrasound, we transfected cells with a combination of mechanosensitive channel MscS and gas vesicle-forming proteins. By decreasing the power of ultrasound stimulation we demonstrated that cells expressing a combination of both ectopic channels and gas vesicles were activated as a result of the ultrasound stimulation (3.3.7.).

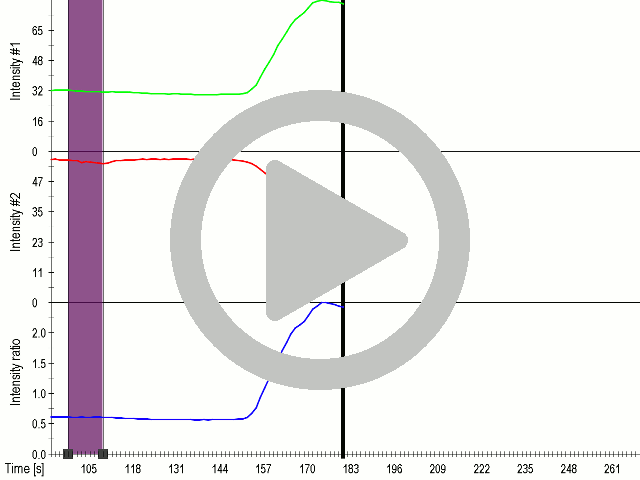

(A) Presentation of the ultrasound stimulation sequence and (B) signal parameters used for stimulation. (C, D) Co-expression of mechanosensitive channels and gas vesicle-forming proteins increased sensitivity to ultrasound stimulation in comparison to the cells without exogenous mechanosensitive channels. HEK293 cells expressing gas vesicle-forming proteins GvpA and GvpC with or without MscS were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. Changes in fluorescence intensity of calcium indicators Fluo-4 (green line) and Fura Red (red line) are shown. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
To prove that calcium influx was the mediator of activation of mechanosensitive channels, we used an inhibitor of ion channels gadolinium (Gd3+), which has high charge density and similar ionic radius to Ca2+ .By blocking the pore of the channel it acts as an general inhibitor of calcium ion channels

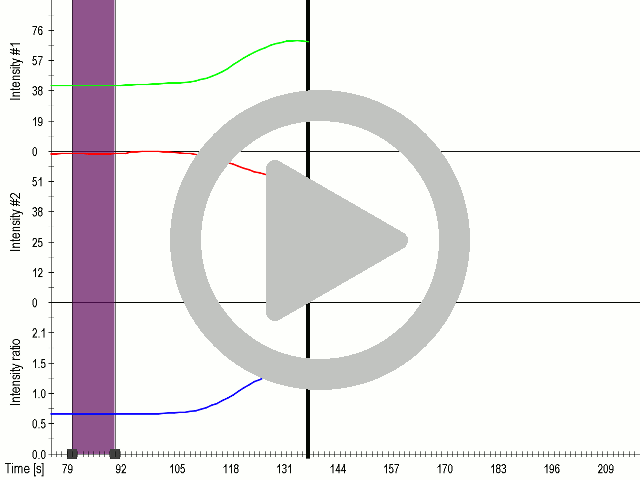


(A) Presentation of the ultrasound stimulation sequence and (B) signal parameters used for stimulation. (C, D) Gadolinium inhibits activation of mechanosensitive ion channels after ultrasound stimulation. HEK293 cells expressing gas vesicle-forming proteins GvpA and GvpC with or without MscS were treated with gadolinium (red line) or not (grey line) and stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D). Changes in fluorescence intensity of calcium indicators Fluo-4 (green line) and Fura Red (red line) are shown. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
This is the first time demonstration that that gas vesicle-forming proteins can be expressed in human cells and that they improve the sensitivity of mechanosensitive channels for ultrasound. Moreover when the gas vesicle-forming proteins were co-expressed with ectopic ion channels the calcium influx could be achieved with low-power ultrasound.
Ultrasound is a type of mechanical stimulus, therefore we reasoned that cells might exhibit response also to other types of stimulus such as the touch. The next task was to couple the response of mechanosensitive channels to the mediator of signaling or to the genetically encoded reporter. Mediator of this activation are Ca2+ ions, which are sensed by different cellular proteins and which have been in the literature detected by several designed sensors. The mechanosensing device was then coupled to the calcium split luciferase reporter based on M13 and calmodulin or can be coupled to the protease split system and response of cells was determined against mechanical stimulus in a Touchpaint implementation.


