Zigapusnik (Talk | contribs) |
|||
| Line 2: | Line 2: | ||
<html> | <html> | ||
<head> | <head> | ||
| − | <title> | + | <title>Mechanosensitive channels</title> |
<link rel="stylesheet" | <link rel="stylesheet" | ||
href="//2016.igem.org/Team:Slovenia/libraries/semantic-min-css?action=raw&ctype=text/css"> | href="//2016.igem.org/Team:Slovenia/libraries/semantic-min-css?action=raw&ctype=text/css"> | ||
| Line 15: | Line 15: | ||
<script type="text/javascript" | <script type="text/javascript" | ||
src="https://2016.igem.org/Team:Slovenia/libraries/bibtexparse-js?action=raw&ctype=text/javascript"></script> | src="https://2016.igem.org/Team:Slovenia/libraries/bibtexparse-js?action=raw&ctype=text/javascript"></script> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</head> | </head> | ||
<body> | <body> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<div id="example"> | <div id="example"> | ||
<div class="pusher"> | <div class="pusher"> | ||
| Line 168: | Line 25: | ||
</a> | </a> | ||
<div class="ui vertical sticky text menu"> | <div class="ui vertical sticky text menu"> | ||
| + | <a class="item" href="//2016.igem.org/Team:Slovenia/Mechanosensing/Overview"> | ||
| + | <i class="chevron circle left icon"></i> | ||
| + | <b>Overview</b> | ||
| + | </a> | ||
<a class="item" href="#intro" style="margin-left: 10%"> | <a class="item" href="#intro" style="margin-left: 10%"> | ||
<i class="selected radio icon"></i> | <i class="selected radio icon"></i> | ||
| − | <b> | + | <b>Achievements</b> |
</a> | </a> | ||
| − | <a class="item" href="# | + | <a class="item" href="#mot" style="margin-left: 10%"> |
<i class="selected radio icon"></i> | <i class="selected radio icon"></i> | ||
| − | <b> | + | <b>Motivation</b> |
</a> | </a> | ||
| − | <a class="item" href="# | + | <a class="item" href="#loc" style="margin-left: 10%"> |
<i class="selected radio icon"></i> | <i class="selected radio icon"></i> | ||
| − | <b> | + | <b>Localization and</b> |
| + | <br/> | ||
| + | <b style="margin-left: 12%">Expression</b> | ||
</a> | </a> | ||
| − | <a class="item" href="# | + | <a class="item" href="#us" style="margin-left: 10%"> |
<i class="selected radio icon"></i> | <i class="selected radio icon"></i> | ||
| − | <b> | + | <b>Ultrasound stimulation</b> |
</a> | </a> | ||
| − | + | <a class="item" href="//2016.igem.org/Team:Slovenia/Mechanosensing/Gas_vesicles"> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | <a class="item" href="//2016.igem.org/Team:Slovenia/ | + | |
<i class="chevron circle right icon"></i> | <i class="chevron circle right icon"></i> | ||
| − | <b> | + | <b>Gas Vesicles</b> |
</a> | </a> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</div> | </div> | ||
| Line 213: | Line 57: | ||
<!-- menu goes here --> | <!-- menu goes here --> | ||
<!-- content goes here --> | <!-- content goes here --> | ||
| − | <div class="main ui citing justified container"> | + | <div> |
| − | + | <div class="main ui citing justified container" style = "margin-left:20%;"> | |
| − | + | <div> | |
| − | + | <h1 class="ui left dividing header"><span id="intro" class="section"> </span>Enhanced | |
| + | Mechanosensitivity by overexpressed <br/>Mechanosensitive Channels</h1> | ||
| + | <div class="ui segment" style="background-color: #ebc7c7; "> | ||
| + | <ul> | ||
| + | <li><b>Ectopically expressed mechanosensitive ion channels MscS and P3:FAStm:TRPC1 | ||
| + | were used to enhance sensitivity of mammalian cells to ultrasound | ||
| + | stimulation.</b> | ||
| + | <li><b>Membrane localization of the mechanosensitive channel TRPC1 was improved by | ||
| + | fusing it with a FAS transmembrane domain, which also led to increased | ||
| + | sensitivity to ultrasound stimulation.</b> | ||
| + | </ul> | ||
| + | </div> | ||
| + | </div> | ||
<div class="ui segment"> | <div class="ui segment"> | ||
| − | <p> | + | <h4><span id="mot" class="section"> </span></h4> |
| − | + | <p>We chose to test two mechanosensitive channels, human nonspecific cation channel TRPC1 | |
| − | + | and bacterial channel MscS, previously described as important receptors involved | |
| − | + | in the response to mechanical stimulation in human and bacteria | |
| − | + | <x-ref>Haswell2011, Ye2013</x-ref> | |
| − | + | . | |
| − | + | </p> | |
| − | + | <div class="ui styled fluid accordion"> | |
| − | <div> | + | <div class="title"> |
| − | <div class=" | + | <i class="dropdown icon"></i> |
| − | < | + | Further explanation ... |
| − | + | </div> | |
| − | + | <div class="content"> | |
| − | + | <p>Transient receptor potential channel 1 (TRPC1) is a human non-specific cation | |
| − | + | channel located at the plasma membrane. It has been previously reported as | |
| − | + | broadly expressed in human tissues where it functions as a store-operating | |
| − | + | calcium channel | |
| − | + | <x-ref>Xu2001</x-ref> | |
| − | + | . It belongs to the TRP superfamily that | |
| − | + | act as tetrameric transmembrane proteins consisting of a domain formed by six | |
| − | + | transmembrane helices, with a pore between S5 and S6 | |
| − | + | <x-ref>Nilius2007</x-ref> | |
| − | + | . | |
| − | + | These helices present the N- and C-termini to the cytoplasm, promoting the | |
| − | + | formation of functional homo- or hetero-tetramers | |
| − | + | <x-ref>Bianchi2007</x-ref> | |
| − | + | . | |
| − | < | + | </p> |
| − | + | <div style="clear:left; width:50%"> | |
| − | + | <figure data-ref="1"> | |
| − | + | <img onclick="resize(this);" | |
| − | + | src="https://static.igem.org/mediawiki/2016/7/7a/T--Slovenia--3.2.2.PNG"> | |
| − | + | <figcaption><b>Structure of a tetrameric homologous TRPv6 channel (<a | |
| − | + | href="http://www.rcsb.org/pdb/explore.do?structureId=5IRX">PDB | |
| − | + | 5IRX</a>) presented from side and top with seen ion pore.</b> | |
| − | + | </figcaption> | |
| − | + | </figure> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</div> | </div> | ||
| − | <div | + | <p>The second channel that we selected is the bacterial mechanosensitive channel |
| − | < | + | MscS. Its role is to mediate turgor regulation in bacteria and it is |
| − | + | activated by changes in osmotic pressure | |
| − | + | <x-ref>Perozo2003</x-ref> | |
| − | + | . It has been previously shown that MscS is a homoheptamer, each subunit is | |
| + | 31kDa in | ||
| + | size and contains three transmembrane helices with the N-terminus facing the | ||
| + | periplasm and the C-terminus embedded in the cytoplasm | ||
| + | <x-ref>Pivetti2003</x-ref> | ||
| + | . | ||
| + | </p> | ||
| + | <div style="clear:left; width:50%"> | ||
| + | <figure data-ref="2"> | ||
| + | <img onclick="resize(this);" | ||
| + | src="https://static.igem.org/mediawiki/2016/d/df/T--Slovenia--3.2.3.PNG"> | ||
| + | <figcaption><b>. Crystal structure of the MscS channel (<a | ||
| + | href="http://www.rcsb.org/pdb/explore.do?structureId=5AJI">PDB | ||
| + | 5AJI</a>) presented from the side view with presented membrane lipids | ||
| + | and from the top with seen ion pore</b></figcaption> | ||
| + | </figure> | ||
</div> | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| − | + | <h1><span class="section"> </span>Results</h1> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | <h1 | + | |
| − | + | ||
<div class="ui segment"> | <div class="ui segment"> | ||
| − | <div class=" | + | <div> |
| − | <p><img src="//2016.igem.org/wiki/images/ | + | <h4><span id="loc" class="section"> </span>Localization and expression</h4> |
| − | alt=" | + | <p>The mechanosensitive TRPC1 channel with each subunit comprising six transmembrane |
| + | helices ( | ||
| + | <ref>3</ref> | ||
| + | A) and the MscS channel with three transmembrane | ||
| + | helices ( | ||
| + | <ref>3</ref> | ||
| + | A) were expressed in HEK293T cells ( | ||
| + | <ref>3</ref> | ||
| + | D). MscS was detected as the 31 kDa band. TRPC1 was observed at 60 kDa, which was | ||
| + | lower t | ||
| + | han expected. We observed that the membrane localization in HEK293 was more evident | ||
| + | for MscS ( | ||
| + | <ref>3</ref> | ||
| + | B) rather than TRPC1 ( | ||
| + | <ref>3</ref> | ||
| + | C). | ||
| + | </p> | ||
| + | <div style="clear:left; width:100%"> | ||
| + | <figure data-ref="3"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/1/1e/T--Slovenia--3.2.2.png"> | ||
| + | <figcaption><b>Localization and expression of mechanosensitive ion channels MscS | ||
| + | and TRPC1. </b><br/> | ||
| + | <p style="text-align:justify">(A) Scheme of bacterial ion channel MscS (upper) and human ion channel TRPC1 | ||
| + | (lower). | ||
| + | (B) Ion channel MscS localized to plasma membrane. (C) TRPC1 predominantly | ||
| + | localized in the ER. | ||
| + | (D) Ion channels MscS and TRPC1 were expressed in HEK293 cells. HEK293 cells | ||
| + | were transfected with plasmids encoding HA tagged MscS or Myc-tagged TRPC1. | ||
| + | Expression by Western blot and localization by confocal microscopy were | ||
| + | analyzed using anti-HA and anti-Myc antibodies, respectively. | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | <p style="clear:both">To improve membrane localization of TRPC1 we fused a FAS | ||
| + | transmembrane domain to TRPC1 ( | ||
| + | <ref>4</ref> | ||
| + | A), since the | ||
| + | transmembrane FAS domain has been very efficient in Jerala lab for the membrane | ||
| + | localization | ||
| + | <x-ref>Majerle2015</x-ref> | ||
| + | . The strategy of adding an | ||
| + | additional transmembrane domain has to own knowledge not been applied before for the | ||
| + | ion channels. The addition of FAS transmembrane domain to the | ||
| + | N-terminus of TRPC1 improved localization of the protein to plasma membrane in | ||
| + | comparison to the unmodified TRPC1. From the confocal microscopy images | ||
| + | of non-permeabilized cells we verified that the modified channel was inserted into | ||
| + | plasma membrane as predicted, since in non-permeabilized cells antibodies | ||
| + | stained the exposed extracellular HA-tag but not the intracellular Myc-tag ( | ||
| + | <ref>4</ref> | ||
| + | B). | ||
| + | </p> | ||
| + | <div style="float:left; width:100%"> | ||
| + | <figure data-ref="4"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/1/13/T--Slovenia--3.2.3.png"> | ||
| + | <figcaption><b>Localization of fusion protein P3:FAStm:TRPC1.</b><br/> | ||
| + | <p style="text-align:justify">(A) Scheme of ion channel P3:FAStm:TRPC1. (B) Ion channel P3:FAStm:TRPC1 was | ||
| + | localized to plasma membrane. HEK293 cells were transfected with | ||
| + | P3:FAStm:TRPC1 plasmid. 24 h after transfection cells were permeabilized | ||
| + | (upper) or non-permeabilized (lower) and stained with antibodies against | ||
| + | HA and Myc-tag. Localization on plasma membrane is shown with arrows. | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | <p style="clear:both">In addition to the improved membrane localization, the FAS | ||
| + | transmembrane domain linked to the TRPC1 presents another advantage. The TRPC1 is an | ||
| + | ion | ||
| + | channel with six transmembrane helices, therefore both the N- and the C-terminus of | ||
| + | the protein are orientated towards the interior of the cell. By addition | ||
| + | of the FAS transmembrane domain, the N-terminus of P3:FAStm:TRPC1 chimera (where P3 | ||
| + | stands for coiled coil, hyperlink to CC page) is exposed in the extracellular | ||
| + | space and could interact with different proteins from outside the cell via the | ||
| + | N-terminal tag. We reasoned that this interaction could be used to achieve a higher | ||
| + | sensitivity to mechanical stimuli.</p> | ||
| + | |||
| + | <p>After we showed that the selected ion channels MscS, TRPC1 and P3:FAStm:TRPC1 are | ||
| + | expressed in HEK293 and localized at the plasma membrane, we further tested their | ||
| + | function as mechanosensors by exposing them to the ultrasound stimulation. | ||
| + | </p> | ||
| + | </div> | ||
| + | <div> | ||
| + | <h4><span id="us" class="section"> </span>Ultrasound stimulation</h4><br/> | ||
| + | <div style="clear:both" class="ui styled fluid accordion"> | ||
| + | <div class="title"> | ||
| + | <i class="dropdown icon"></i> | ||
| + | Further explanation ... | ||
| + | </div> | ||
| + | <div class="content"> | ||
| + | <p> | ||
| + | Ultrasound stimulation offers potentially remarkable advantages over the | ||
| + | majority of external stimuli used for targeted cell stimulation. | ||
| + | Optogenetics as another | ||
| + | promising approach to cell stimulation requires invasive surgery to | ||
| + | implement optical fibers connected to the source of light – LED or laser | ||
| + | <x-ref>Warden2014</x-ref> | ||
| + | in order to target cells in tissue to activate or silence them. On the other | ||
| + | hand, ultrasound offers a non-invasive approach to overcome the problems | ||
| + | which appear in | ||
| + | the abovementioned method. Its use has been demonstrated potentially even | ||
| + | for noninvasive ultrasound therapy through an intact skull | ||
| + | <x-ref>Hynynen1998</x-ref> | ||
| + | . | ||
| + | Previously, ultrasound had been used in several in vitro studies to directly | ||
| + | stimulate clusters of neurons but also in few model organisms (among others | ||
| + | <x-ref>King2013</x-ref> | ||
| + | . | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | <br/> | ||
| + | <p>For mechanical stimulation of cells with ultrasound we designed our own unique | ||
| + | experimental setup, which included the | ||
| + | <a href="https://2016.igem.org/Team:Slovenia/Hardware">ultrasound device MODUSON</a> | ||
| + | that we constructed connected to the unfocused transducer Olympus | ||
| + | V318-SU and a 3D printed support for a transducer to fix it at a defined position | ||
| + | relative to the cells. Stimulation conditions were optimized for our cell | ||
| + | line and experimental setup. To measure the changes of free calcium ion | ||
| + | concentration we stained cells with two fluorescent dyes Fura Red and Fluo-4. | ||
| + | The combination of these two dyes enabled us to present changes in the calcium ion | ||
| + | concentration as a ratio of the fluorescence intensity at two wavelengths, | ||
| + | which was superior to the intensity based measurements, since it is independent of | ||
| + | photobleaching and dye sequestration | ||
| + | </p> | ||
| + | |||
| + | <div class="ui styled fluid accordion"> | ||
| + | <div class="title"> | ||
| + | <i class="dropdown icon"></i> | ||
| + | Further explanation ... | ||
| + | </div> | ||
| + | <div class="content"> | ||
| + | <p>Fura Red and Fluo-4 are visible light-excitable dyes used for ratiometric | ||
| + | measurement of calcium ions which excitation maximum is at 488 nm. | ||
| + | While Fluo-4 exhibits an increase in fluorescence emission at 515 nm upon | ||
| + | binding of calcium ions, fluorescence emission at 655 nm of Fura Red | ||
| + | decreases once the indicator binds calcium ions. By calculating the ratio of | ||
| + | fluorescence emission intensities captured at 488 nm exaction | ||
| + | (where the difference of fluorescence between the bound and free indicator | ||
| + | is at its maximum), we could observe changes in intracellular | ||
| + | calcium concentrations in real time. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | <br/> | ||
| + | |||
| + | <p>We followed changes of calcium concentration after ultrasound stimulation in real | ||
| + | time using ratiometric confocal microscopy. For processing of data we | ||
| + | developed our software <a | ||
| + | href="https://2016.igem.org/Team:Slovenia/Software">CaPTURE</a>, which | ||
| + | automatically calculated the ratio between fluorescence | ||
| + | intensities of FuraRed and Fluo-4 and presented the data as image and calculated | ||
| + | values. | ||
| + | </p> | ||
| + | |||
| + | <p>We showed that by expressing the MscS channel, cells gained sensitivity for | ||
| + | ultrasound stimulation in comparison to non-transfected cells ( | ||
| + | <ref>6</ref> | ||
| + | ). | ||
| + | Influx of calcium ions was observed at a lower rate in the case of ectopically | ||
| + | expressed TRPC1 (data not shown), probably due to its poor membrane localization. | ||
| + | </p> | ||
| + | |||
| + | <div style="clear:both; width:50%" align="center"> | ||
| + | <figure data-ref="5"> | ||
| + | <img src=" https://static.igem.org/mediawiki/2016/f/f2/T--Slovenia--S.3.1.1.png "> | ||
| + | <figcaption><b>INSERT!!!</b><br/></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | <div style="clear:both;" align="center"> | ||
| + | <figure data-ref="6"> | ||
| + | <img class="ui big centered image" | ||
| + | src="https://static.igem.org/mediawiki/2016/2/27/T--Slovenia--3.2.5.png"> | ||
| + | <div style="clear:both; display:block; width: 90%; margin-right: auto; margin-left: auto"> | ||
| + | <div style="display: block; float: left; width: 80%;"> | ||
| + | <!-- experiment: 20161002 2 MscS + Gvp 200V 90s --> | ||
| + | <img class="playme" id="gifGroup8" | ||
| + | src="//2016.igem.org/wiki/images/a/ad/T--Slovenia--Group71.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/b/be/T--Slovenia--20160929_2_MscS_200V_110s_graf.gif"> | ||
| + | </div> | ||
| + | <div> | ||
| + | <!-- experiment: 20161002 2 MscS + Gvp 200V 90s --> | ||
| + | <div style="display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup8" | ||
| + | src="//2016.igem.org/wiki/images/7/70/T--Slovenia--Group72.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/8/84/T--Slovenia--20160929_2_MscS_200V_110s_Regions.gif"> | ||
| + | </div> | ||
| + | <div style="display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup8" | ||
| + | src="//2016.igem.org/wiki/images/8/83/T--Slovenia--Group73.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/8/8f/T--Slovenia--20160929_2_MscS_200V_110s_Controls.gif"> | ||
| + | </div> | ||
| + | <div style="display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup8" | ||
| + | src="//2016.igem.org/wiki/images/0/0e/T--Slovenia--Group74.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/e/e4/T--Slovenia--20160929_2_MscS_200V_110s_HeatMap.gif"> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <figcaption><b> MscS channel improves sensitivity of cells for | ||
| + | ultrasound.</b><br/> | ||
| + | <p style="text-align:justify">(A) Schematic representation of a stimulation sequence and (B) signal | ||
| + | parameters used for stimulation. | ||
| + | (C) and (D )Cells expressing MscS showed increased sensitivity to ultrasound | ||
| + | stimulation in comparison to the cells without exogenous mechanosensitive | ||
| + | channel. HEK293 cells expressing MscS channels or control cells transfected | ||
| + | with vector were stimulated with ultrasound for 10 s and calcium influx | ||
| + | was recorded in real time (D) using a confocal microscope. For comparison | ||
| + | cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red | ||
| + | dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio | ||
| + | (blue line) was calculated from fluorescence intensities of Fura Red and | ||
| + | Fluo-4 | ||
| + | using <a href="https://2016.igem.org/Team:Slovenia/Software">CaPTURE</a>. | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <p style="clear:both">Fusion of the FAS transmembrane domain to TRPC1 did not only | ||
| + | improve its membrane localization, but also significantly enhanced its sensitivity | ||
| + | to ultrasound | ||
| + | stimulation ( | ||
| + | <ref>8</ref> | ||
| + | C), suggesting the importance of membrane localization in the function of | ||
| + | mechanosensors. | ||
| + | </p> | ||
| + | |||
| + | <div style="clear:left;"> | ||
| + | <figure data-ref="7"> | ||
| + | <img onclick="resize(this);" class="ui medium image" | ||
| + | src=" https://static.igem.org/mediawiki/2016/f/fd/T--Slovenia--S.3.1.2.png"> | ||
| + | <figcaption><b>INSERT!!!</b><br/></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | <div style="clear:both;" align="center"> | ||
| + | <figure data-ref="8"> | ||
| + | <img class="ui big centered image" | ||
| + | src="https://static.igem.org/mediawiki/2016/8/8a/T--Slovenia--3.2.4.png"> | ||
| + | <div style="clear:both; display:block; width: 90%; margin-right: auto; margin-left: auto"> | ||
| + | <div style="display: block; float: left; width: 80%;"> | ||
| + | <!-- experiment: 20161002 2 MscS + Gvp 200V 90s --> | ||
| + | <img class="playme" id="gifGroup9" | ||
| + | src="//2016.igem.org/wiki/images/1/10/T--Slovenia--Group51.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/4/41/T--Slovenia--201607192_P3_FAS_TRPC1200V.gif"> | ||
| + | </div> | ||
| + | <div> | ||
| + | <!-- experiment: 20161002 2 MscS + Gvp 200V 90s --> | ||
| + | <div style="display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup9" | ||
| + | src="//2016.igem.org/wiki/images/c/c7/T--Slovenia--Group52.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/0/00/T--Slovenia--201607192_P3_FAS_TRPC1200Vregion.gif"> | ||
| + | </div> | ||
| + | <div style="display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup9" | ||
| + | src="//2016.igem.org/wiki/images/b/be/T--Slovenia--Group53.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/6/60/T--Slovenia--201607192_P3_FAS_TRPC1200Vcontrol.gif"> | ||
| + | </div> | ||
| + | <div style="display: block; float: left; width: 20%;"> | ||
| + | <img class="gifGroup9" | ||
| + | src="//2016.igem.org/wiki/images/c/cb/T--Slovenia--Group54.png" | ||
| + | width="100%" | ||
| + | data-alt="//2016.igem.org/wiki/images/b/b9/T--Slovenia--201607192_P3_FAS_TRPC1200Vheatmap.gif"> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <figcaption><b>P3:FAS:TRPC1 channel improves sensitivity of cells for | ||
| + | ultrasound.</b><br/> | ||
| + | <p style="text-align:justify">(A) Schematic presentation of a stimulation sequence and (B) signal | ||
| + | parameters used for stimulation. | ||
| + | (C) and (D ) Cells expressing P3:FAS:TRPC1 showed increased sensitivity to | ||
| + | ultrasound stimulation in comparison to the cells without exogenous | ||
| + | mechanosensitive channel. | ||
| + | HEK293 cells expressing P3:FAS:TRPC1 were stimulated with ultrasound for 10 | ||
| + | s and calcium influx was recorded in real time (D) using a confocal | ||
| + | microscope. For comparison | ||
| + | cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red | ||
| + | dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio | ||
| + | (blue line) was calculated | ||
| + | from fluorescence intensities of Fura Red and Fluo-4 using <a | ||
| + | href="https://2016.igem.org/Team:Slovenia/Software">CaPTURE</a>. | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <p style="clear:both">In order to observe mechanostimulation of cells with ectopically | ||
| + | expressed mechanoreceptors we had to use high-power ultrasound, however we tested | ||
| + | that the cells nevertheless | ||
| + | did not lose the viability by ultrasound stimulation. Our next challenge was to | ||
| + | further improve sensitivity of cells to respond to lower power ultrasound as this | ||
| + | would avoid | ||
| + | stimulation of any endogenous channels and limit stimulation only to the engineered. | ||
</p> | </p> | ||
| − | |||
| − | |||
</div> | </div> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</div> | </div> | ||
</div> | </div> | ||
| Line 535: | Line 449: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | |||
| + | <script> | ||
| + | $(document).ready(function () { | ||
| + | var getGif = function () { | ||
| + | var gif = []; | ||
| + | $('img').each(function () { | ||
| + | console.log("image detected"); | ||
| + | var data = $(this).data('alt'); | ||
| + | gif.push(data); | ||
| + | }); | ||
| + | return gif; | ||
| + | }; | ||
| + | var gif = getGif(); | ||
| + | //preload images | ||
| + | var image = []; | ||
| + | $.each(gif, function (index) { | ||
| + | console.log("loading resource"); | ||
| + | image[index] = new Image(); | ||
| + | image[index].src = gif[index]; | ||
| + | }); | ||
| + | $('.playme').on('click', function () { | ||
| + | console.log("click detected"); | ||
| + | var sel = '.'.concat($(this).attr("id")); | ||
| + | console.log(sel); | ||
| + | var parent = $(this); | ||
| + | var parAlt = parent.attr('data-alt'); | ||
| + | var parSrc = parent.attr('src'); | ||
| + | parent.attr("src", parAlt).attr("data-alt", parSrc); | ||
| + | $(sel).each(function () { | ||
| + | console.log("anyone"); | ||
| + | var img = $(this); | ||
| + | var imgSrc = img.attr('src'); | ||
| + | var imgAlt = img.attr('data-alt'); | ||
| + | img.attr("src", imgAlt).attr("data-alt", imgSrc); | ||
| + | }); | ||
| + | }); | ||
| + | }); | ||
| + | </script> | ||
</body> | </body> | ||
</html> | </html> | ||
Revision as of 02:32, 18 October 2016
Enhanced
Mechanosensitivity by overexpressed
Mechanosensitive Channels
- Ectopically expressed mechanosensitive ion channels MscS and P3:FAStm:TRPC1 were used to enhance sensitivity of mammalian cells to ultrasound stimulation.
- Membrane localization of the mechanosensitive channel TRPC1 was improved by fusing it with a FAS transmembrane domain, which also led to increased sensitivity to ultrasound stimulation.
We chose to test two mechanosensitive channels, human nonspecific cation channel TRPC1
and bacterial channel MscS, previously described as important receptors involved
in the response to mechanical stimulation in human and bacteria
Transient receptor potential channel 1 (TRPC1) is a human non-specific cation
channel located at the plasma membrane. It has been previously reported as
broadly expressed in human tissues where it functions as a store-operating
calcium channel

The second channel that we selected is the bacterial mechanosensitive channel
MscS. Its role is to mediate turgor regulation in bacteria and it is
activated by changes in osmotic pressure

Results
Localization and expression
The mechanosensitive TRPC1 channel with each subunit comprising six transmembrane helices ( 3 A) and the MscS channel with three transmembrane helices ( 3 A) were expressed in HEK293T cells ( 3 D). MscS was detected as the 31 kDa band. TRPC1 was observed at 60 kDa, which was lower t han expected. We observed that the membrane localization in HEK293 was more evident for MscS ( 3 B) rather than TRPC1 ( 3 C).

(A) Scheme of bacterial ion channel MscS (upper) and human ion channel TRPC1 (lower). (B) Ion channel MscS localized to plasma membrane. (C) TRPC1 predominantly localized in the ER. (D) Ion channels MscS and TRPC1 were expressed in HEK293 cells. HEK293 cells were transfected with plasmids encoding HA tagged MscS or Myc-tagged TRPC1. Expression by Western blot and localization by confocal microscopy were analyzed using anti-HA and anti-Myc antibodies, respectively.
To improve membrane localization of TRPC1 we fused a FAS
transmembrane domain to TRPC1 (
4
A), since the
transmembrane FAS domain has been very efficient in Jerala lab for the membrane
localization

(A) Scheme of ion channel P3:FAStm:TRPC1. (B) Ion channel P3:FAStm:TRPC1 was localized to plasma membrane. HEK293 cells were transfected with P3:FAStm:TRPC1 plasmid. 24 h after transfection cells were permeabilized (upper) or non-permeabilized (lower) and stained with antibodies against HA and Myc-tag. Localization on plasma membrane is shown with arrows.
In addition to the improved membrane localization, the FAS transmembrane domain linked to the TRPC1 presents another advantage. The TRPC1 is an ion channel with six transmembrane helices, therefore both the N- and the C-terminus of the protein are orientated towards the interior of the cell. By addition of the FAS transmembrane domain, the N-terminus of P3:FAStm:TRPC1 chimera (where P3 stands for coiled coil, hyperlink to CC page) is exposed in the extracellular space and could interact with different proteins from outside the cell via the N-terminal tag. We reasoned that this interaction could be used to achieve a higher sensitivity to mechanical stimuli.
After we showed that the selected ion channels MscS, TRPC1 and P3:FAStm:TRPC1 are expressed in HEK293 and localized at the plasma membrane, we further tested their function as mechanosensors by exposing them to the ultrasound stimulation.
Ultrasound stimulation
Ultrasound stimulation offers potentially remarkable advantages over the
majority of external stimuli used for targeted cell stimulation.
Optogenetics as another
promising approach to cell stimulation requires invasive surgery to
implement optical fibers connected to the source of light – LED or laser
For mechanical stimulation of cells with ultrasound we designed our own unique experimental setup, which included the ultrasound device MODUSON that we constructed connected to the unfocused transducer Olympus V318-SU and a 3D printed support for a transducer to fix it at a defined position relative to the cells. Stimulation conditions were optimized for our cell line and experimental setup. To measure the changes of free calcium ion concentration we stained cells with two fluorescent dyes Fura Red and Fluo-4. The combination of these two dyes enabled us to present changes in the calcium ion concentration as a ratio of the fluorescence intensity at two wavelengths, which was superior to the intensity based measurements, since it is independent of photobleaching and dye sequestration
Fura Red and Fluo-4 are visible light-excitable dyes used for ratiometric measurement of calcium ions which excitation maximum is at 488 nm. While Fluo-4 exhibits an increase in fluorescence emission at 515 nm upon binding of calcium ions, fluorescence emission at 655 nm of Fura Red decreases once the indicator binds calcium ions. By calculating the ratio of fluorescence emission intensities captured at 488 nm exaction (where the difference of fluorescence between the bound and free indicator is at its maximum), we could observe changes in intracellular calcium concentrations in real time.
We followed changes of calcium concentration after ultrasound stimulation in real time using ratiometric confocal microscopy. For processing of data we developed our software CaPTURE, which automatically calculated the ratio between fluorescence intensities of FuraRed and Fluo-4 and presented the data as image and calculated values.
We showed that by expressing the MscS channel, cells gained sensitivity for ultrasound stimulation in comparison to non-transfected cells ( 6 ). Influx of calcium ions was observed at a lower rate in the case of ectopically expressed TRPC1 (data not shown), probably due to its poor membrane localization.


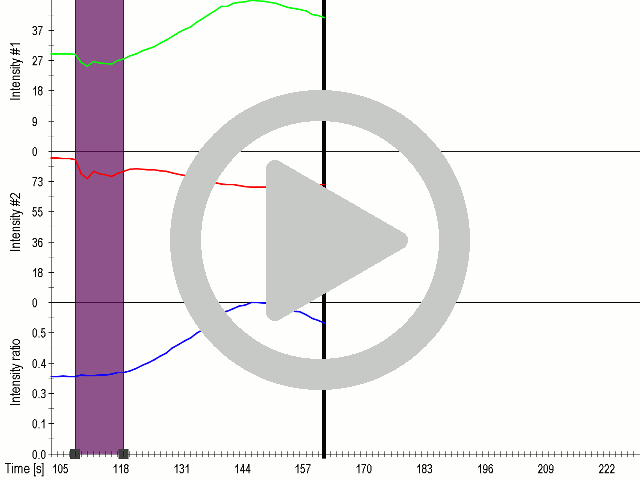
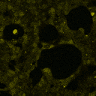
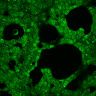

(A) Schematic representation of a stimulation sequence and (B) signal parameters used for stimulation. (C) and (D )Cells expressing MscS showed increased sensitivity to ultrasound stimulation in comparison to the cells without exogenous mechanosensitive channel. HEK293 cells expressing MscS channels or control cells transfected with vector were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. For comparison cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
Fusion of the FAS transmembrane domain to TRPC1 did not only improve its membrane localization, but also significantly enhanced its sensitivity to ultrasound stimulation ( 8 C), suggesting the importance of membrane localization in the function of mechanosensors.


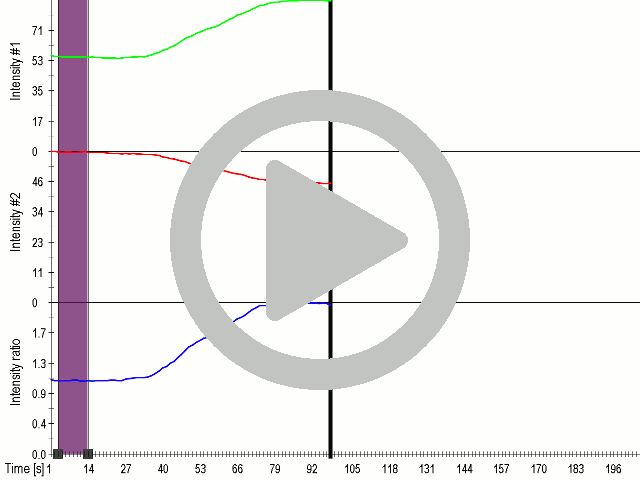
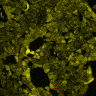


(A) Schematic presentation of a stimulation sequence and (B) signal parameters used for stimulation. (C) and (D ) Cells expressing P3:FAS:TRPC1 showed increased sensitivity to ultrasound stimulation in comparison to the cells without exogenous mechanosensitive channel. HEK293 cells expressing P3:FAS:TRPC1 were stimulated with ultrasound for 10 s and calcium influx was recorded in real time (D) using a confocal microscope. For comparison cells without ectopic MscS were used. Fluo-4 (D, green line) and Fura Red dyes (D, red line) were used for ratiometric calcium imaging. (D) Ratio (blue line) was calculated from fluorescence intensities of Fura Red and Fluo-4 using CaPTURE.
In order to observe mechanostimulation of cells with ectopically expressed mechanoreceptors we had to use high-power ultrasound, however we tested that the cells nevertheless did not lose the viability by ultrasound stimulation. Our next challenge was to further improve sensitivity of cells to respond to lower power ultrasound as this would avoid stimulation of any endogenous channels and limit stimulation only to the engineered.


