(→The Problem with current Platinum Extraction) |
(→The Problem with current Platinum Extraction) |
||
| Line 31: | Line 31: | ||
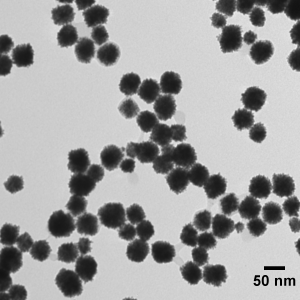
==The Problem with current Platinum Extraction== | ==The Problem with current Platinum Extraction== | ||
| − | [[File:T--Aix-Marseille--extraction.jpeg|400px| | + | [[File:T--Aix-Marseille--extraction.jpeg|400px|right]] |
Platinum is a rare metal because there are only a few mines in the world where it can be obtained. And even then it is usually only a byproduct of nickel and copper mining occurring in concentrations close to 2 g/t. During the process of extraction huge amounts of hydrochloric and sulfuric acid are used to eliminate impurities. | Platinum is a rare metal because there are only a few mines in the world where it can be obtained. And even then it is usually only a byproduct of nickel and copper mining occurring in concentrations close to 2 g/t. During the process of extraction huge amounts of hydrochloric and sulfuric acid are used to eliminate impurities. | ||
Project Description
If you are looking for information about our improved part, go here.
Video presentation
The Problem
- Platinum is an essential natural resource with no real method of extraction than destructive mining methods.
- Platinum resources are draining and if recycling methods are not developed, the world could run out of platinum in the upcoming 50 years.
- As a result of emissions from automobile catalytic converters there are high concentrations of platinum deposited in the soil next to highways and big roads, often higher than in mines.
- There is an issue with industrial wastewater treatment, minerals and metals accumulate so much that companies discard it instead of using decontamination treatment. Platinum concentrations are high in plants cause of the treatment of rainwater which is charged with metals et minerals.
Platinum is an Essential Metal
Platinum is a dense, malleable, ductile, precious and highly unreactive transition metal. Thanks to its catalytic properties and remarkable resistance to corrosion even in high temperatures, it is a highly desired metal.
Platinum is widely used in laboratory equipment, platinum resistance thermometers, electrical contacts and electrodes, medical equipment, chemotherapy, jewelry and etc.
But most importantly platinum is used as the main catalyst in catalytic converters. In fact since the 90s a legislation has been passed making platinum containing catalytic converters mandatory for all cars in most parts of the world. Here, the platinum catalyses oxidation and reduction reactions that neutralize toxic gases emitted during combustion.
The Problem with current Platinum Extraction
Platinum is a rare metal because there are only a few mines in the world where it can be obtained. And even then it is usually only a byproduct of nickel and copper mining occurring in concentrations close to 2 g/t. During the process of extraction huge amounts of hydrochloric and sulfuric acid are used to eliminate impurities.
Most importantly, platinum resources are draining. Experts have estimated that in the year 2064 all known economically workable platinum deposits will be exhausted. It will be necessary to exploit other sources of platinum and develop viable recycling methods.
Secondly, there are no current methods of recycling platinum from accumulations in the environment that have been shown to work.
Finally, there are also various socio medical complications associated with platinum mining. Miners and workers are usually exposed to unsafe chemical substances and toxic fumes. Safety measures are often disregarded. An alternative solution to mining could help in the resolution of this issue.
Our Solution using synthetic biology
- Concentrate the platinum accumulated in plants because of phytoremediation or in sludge in rainwater treatment centers.
- Purify the Platinum from other metals or inorganic compounds.
- Transform it into a highly desirable form - nanoparticles.
It is hard to imagine applying modern industrial methods as a solution for recycling the platinum in the soil of big highways. It would most certainly be very pollutive and a waste of energy, resources and time.
But we think a biological solution is possible. We thought, that after all bacteria have evolutionary adaptations that allow them to internalise metals. If we could exploit these systems and target platinum specifically enough, we could find a durable, simple, cheap and environmentally friendly solution.
The main goal of our project is to concentrate the platinum as much as possible.
The first step relies on the affinity of small organic molecules, siderophores, to bind solubilized Platinum atoms and thus favor the further solubilisation of more platinum compounds. We accomplish this by inserting a plasmid containing an operon with the four enzymes (Des A, Des B, Des C, Des D) necessary to synthesise our siderophore - Desferrioxamine B, into E. coli.
As a second level of concentrating the platinum even more, we plan to use the principle of biosorption. A modified FliC protein complex will be cloned into E. coli and enable the flagella of the bacterium so that it can bind platinum atoms. This specificity will be possible thanks to a recently discovered peptide that will be inserted into the sequence of the FliC protein. The benefit of using the biosorption is to obtain nanoparticles of platinum, a highly valuable form of the metal.