(→Pathway demonstration) |
(→BBa_K1951011 : Desferrioxamine B pathway producer in Streptomyces coelicolor) |
||
| Line 41: | Line 41: | ||
</div> | </div> | ||
| + | |||
Siderophores are small, high-affinity iron chelating compounds secreted by microorganisms such as bacteria, fungi and grasses. Siderophores are amongst the strongest soluble Fe3+ binding agents known. | Siderophores are small, high-affinity iron chelating compounds secreted by microorganisms such as bacteria, fungi and grasses. Siderophores are amongst the strongest soluble Fe3+ binding agents known. | ||
Contents
- 1 Mobilisation by a Siderophore
- 2 Biosorption parts
Composite part
Mobilisation by a Siderophore
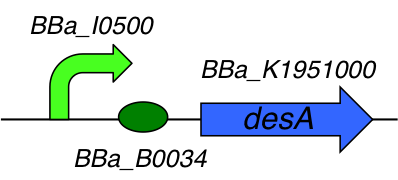
[http://parts.igem.org/Part:BBa_K1951004 BBa_K1951004] : DesA producer
Part Composition:
This part is composed of 3 subparts :
- [http://parts.igem.org/Part:BBa_I0500 I0500] : pARA/araC inducible promoter
- [http://parts.igem.org/Part:BBa_B0034 B0034] : Ribosome Binding Site
- [http://parts.igem.org/Part:BBa_K1951000 K1951000] : Lysine decarboxylase
Lysine decarboxylase DesA :
This DNA sequence codes a [http://metacyc.org/gene?orgid=META&id=SCO2782 Lysine decarboxylase] (Streptomyces coelicolor) which is an enzyme from the lyase family that converts lysine into cadaverine. The enzyme releases the carbonyl group of the lysin amino acid. Cadaverine (or 1,5-diaminopentane) is a primary diamine which renders the medium alkaline. The lysine decarboxylase is an enzyme whose synthesis is promoted by anaerobiosis and an acidic pH. This enzyme is also the first step in the production of desferrioxame B which is a siderophore.
We succeed to produce DesA under an Arabinose indcution.
We registered the original sequence of this subpart in the iGEM registry of standard parts ([http://parts.igem.org/Part:BBa_K1951000 BBa_K1951000]). We optimized our sequence for E. coli and ordered the synthesis by addition of an inducible promoter.
As you can see in [http://parts.igem.org/Part:BBa_K1951004 the registry] all our expectations on this BioBrick were validated and it worked fine.
[http://parts.igem.org/Part:BBa_K1951011 BBa_K1951011] : Desferrioxamine B pathway producer in Streptomyces coelicolor
The purpose of this biobrick is to produce desferrioxamine B siderophore by a controled manner, for controlled potential toxicity. This is also to reconstruct the full pathway of this siderophore production.
This biobrick was made from 6 parts:
Available in the registry :
- [http://parts.igem.org/Part:BBa_I0500 BBa_I0500]
- [http://parts.igem.org/Part:BBa_B0034 BBa_B0034]
Designed by our team :
- [http://parts.igem.org/Part:BBa_K1951000 BBa_K1951000]
- [http://parts.igem.org/Part:BBa_K1951001 BBa_K1951001]
- [http://parts.igem.org/Part:BBa_K1951002 BBa_K1951002]
- [http://parts.igem.org/Part:BBa_K1951003 BBa_K1951003]
Siderophores are small, high-affinity iron chelating compounds secreted by microorganisms such as bacteria, fungi and grasses. Siderophores are amongst the strongest soluble Fe3+ binding agents known.
A precious metal binder
Previous work showed that siderophores are able to catch other metals by default. Especifically, many articles showed a high affinity of Desferrioxamine B (produce by Streptomyces coelicolor) for tetravalent metal ions like platinum. These results encouraged us to make a biobrick coding the sequence corresponding to the 4 enzymes involved on the metabolic pathway of Desferrioxamine B. E. coli was used to produce this biobrick. Produce a gram positive bacteria pathway in a gram negative bacteria is restrictive [1] , considering the risk of toxicity for themselves. To counter this potential issue, we regulate transcription using the control of an inductible promotor (pBAD/araC).
A medical treatment
Desferrioxamine B is a really well referenced siderophore.
Deferoxamine acts by binding free iron in the bloodstream and enhancing its elimination in the urine. By removing excess iron, the agent reduces the damage done to various organs and tissues, such as the liver. Also, it speeds healing of nerve damage (and minimizes the extent of recent nerve trauma).
- It is currently used of on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system. But it is used in the treatment of many others diseases as well.
- Desferrioxamine B may modulate expression and release of inflammatory mediators by specific cell types.
Pathway demonstration
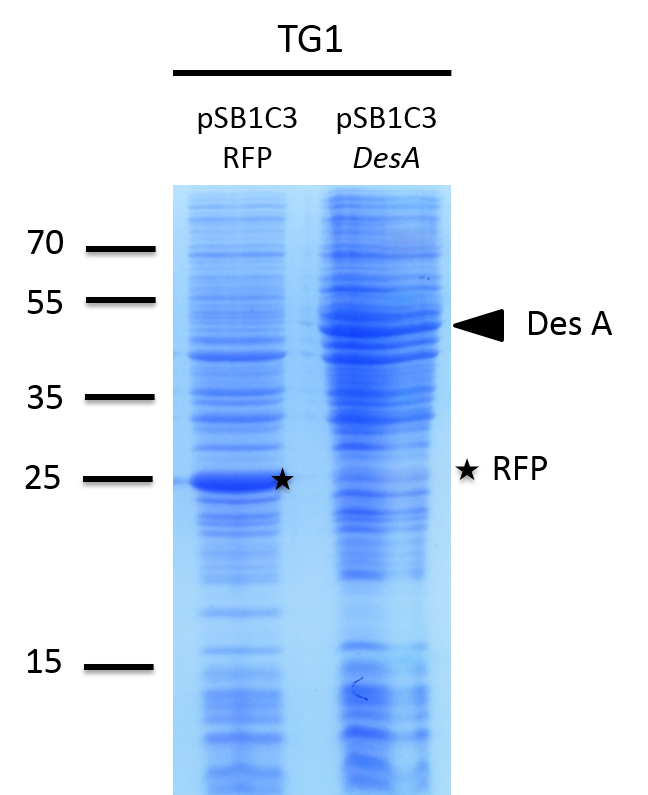
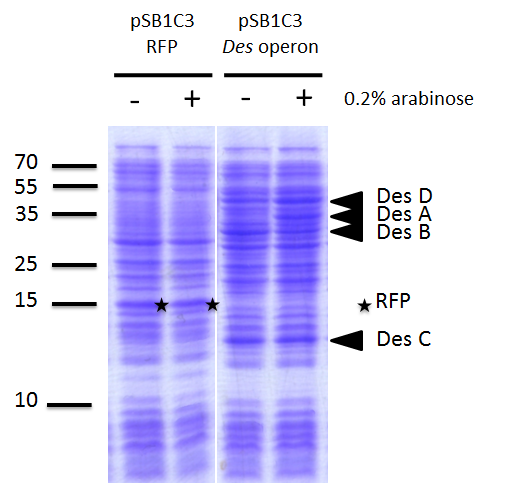
We investigated if the DesA, DesB, DesC and DesD proteins were well produced by our biobrick [http://parts.igem.org/Part:BBa_K1951011 BBa_K1951011] using SDS PAGE.
To do this we performed SDS PAGE (protocol) and stained with coomassie blue using cells containing this biobrick. From an over night starter, cells were diluted and grown from Abs(600nm)=0.2 to Abs(600nm)=1. Then 1UOD of cells (1.67ml at 0.6OD) was collected and centrifuged at 5000g for 5min. After removal of the supernatant, the cell pellet was resuspended in 50µL SDS-PAGE sample buffer. We heated the mix at 95°C during 15min. The sample was loaded onto a polyacrylamide gel and migrated during 50min at 180V. Staining was done using coomassie blue.
We compared the production of proteins in different background :
- E. coli TG1 strain with pSB1C3 containing the RFP coding sequence (negative control)
- E. coli TG1 strain with des operon ([http://parts.igem.org/Part:BBa_K1951011 BBa_K1951011]) before and after induction. (You can observe the production of the 4 proteins on the figure on the right; left : before induction, right : after induction)
Results showed the production of the 4 proteins DesA, DesB, DesC and DesD, all involved in the desferrioxamine B biosynthesis pathway. We can notice that a leak of the promoter pBAD, indeed we note the production of Des proteins without arabinose induction.
Biosorption parts
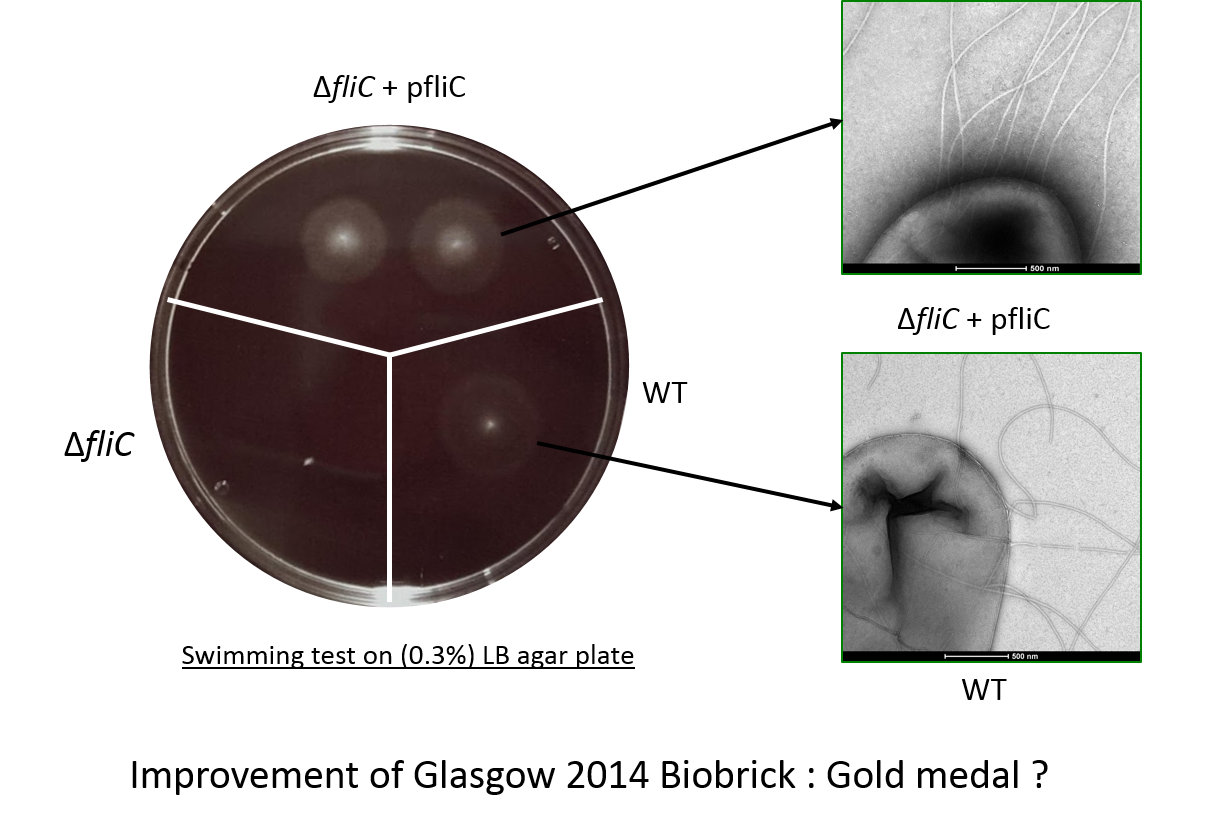
[http://parts.igem.org/Part:BBa_K1951008 BBa_K1951008] : FliC E. coli producer
We also improved an existing BioBrick, Glasgow 2014 team's BioBrick ([http://parts.igem.org/Part:BBa_K1463604 BBa K1463604]), making our brick ([http://parts.igem.org/Part:BBa_K1951008 BBa K1951008]). This BioBrick is an improvement of the orignial BioBrick in three ways:
- The codon usage has been optimized to ensure a strong expression in E.coli,
- The RBS has no mutation so the flagellin is well expressed,
- Our swimming test show that the swimming phenotype of the BioBick is improved thanks to our changes.
All the functional tests and experiments, show this part is functional, are listed here.
Part Composition:
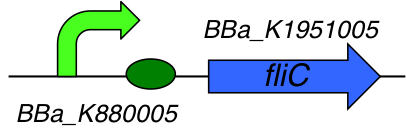
This part is a composite part composed of 2 Biobricks :
- [http://parts.igem.org/Part:BBa_K1951005 BBa_K1951005] : Flagellin C
- [http://parts.igem.org/Part:BBa_K880005 BBa_K880005] : Strong promoter, strong RBS combination
Flagellin C from Escherichia coli
Flagellin C (FliC) protein from Escherichia coli strain is the main protein constitutive of the flagelar filament and is involved to promote bacterial swimming. This sequence is conserved in many bacterial strains. It has been demonstrated that flagellin has the ability to adsorb precious metal such as platinum or gold. We made a FliC mutant by transduction using phage P1 in a E. Coli W3110 strain. Then we have complemented the fliC mutant W3110 with [http://parts.igem.org/Part:BBa_K1951008 BBa_K1951008] and performed a swimming test for every background. The result has shown that swimming was recovered into the complemented fliC mutant W3110
Flagellin C swimming test
We constructed a fliC deletion mutant that is unable to swim, from the wild-type strain W3110. The ability to swim was restored to this mutant by our biobrick, as can be seen in the illustration here. This demonstrated that the protein can be correctly inserted into flagella and functions.
Part Assembly:
The subparts were assembled using standard BioBrick Assembly.
[http://parts.igem.org/Part:BBa_K1941009 BBa_K1941009] : FliC Desulfovibrio vulgaris producer
General
Flagellin (FliC) protein from Desulfovibrio vulgaris strain is the main protein constitutive of the flagellum filament and is involved in bacterial swimming. This protein is conserved in many bacterial strains as the capacity of swimming given by the flagellum confers a great selective advantage.
Metal Biosorption Capacity
It has been demonstrated that Flagellin has the ability to adsorb precious metal on its surface such as platinum, palladium gold [2][3] and this was important for our project Highway to platinum
Immune response capacity
The propensity of the immune response to flagellin may be explained by two facts:
- Flagellin is an extremely abundant protein in flagellated bacteria.
- There exists a specific innate immune receptor that recognizes flagellin, Toll-like receptor 5 (TLR5). [4]
[http://parts.igem.org/Part:BBa_K19410010 BBa_K1941010] : CsgA Escherichia coli producer
CsgA is the major structural subunit of the curli fimbriae. Curli are coiled surface structures that assemble preferentially at growth temperatures below 37 degrees Celsius. Curli are the major proteinaceous component of a complex extracellular matrix produced by many Enterobacteriaceae. Curli were first discovered in the late 1980s on Escherichia coli strains that caused bovine mastitis, and have since been implicated in many physiological and pathogenic processes of E. coli and Salmonella spp. Curli fibers are involved in adhesion to surfaces, cell aggregation, and biofilm formation. Curli also mediate host cell adhesion and invasion, and they are potent inducers of the host inflammatory response. The biobrick contains a strong promotor.
- ↑ Wandersman & Delepaire https://www.ncbi.nlm.nih.gov/pubmed/15487950
- ↑ Deplanche & al., 2007 http://onlinelibrary.wiley.com/doi/10.1002/bit.21688/abstract.
- ↑ Capeness & al. 2015, http://eprints.nottingham.ac.uk/27979/1/Michael%20Capeness%20-%20Thesis%20-%20PDF.pdf
- ↑ Kathrani A. & al, 2012 http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0030117)