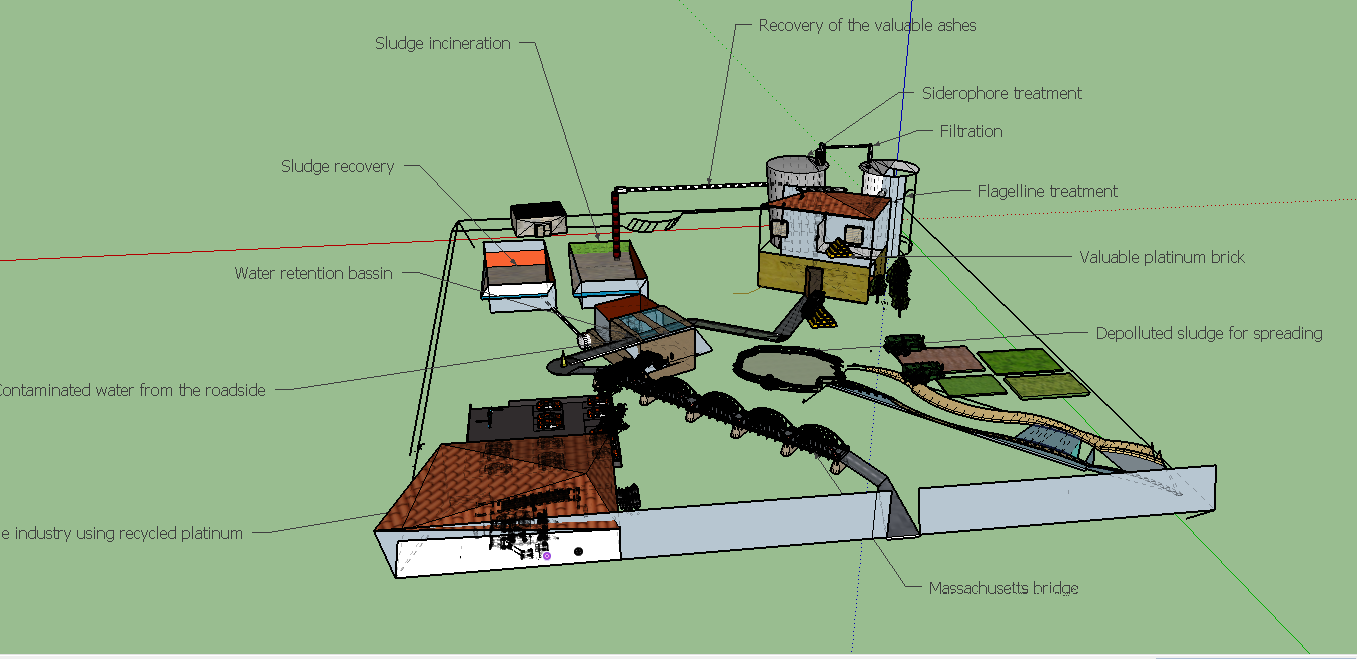
Our process
In this page we developed all estimation and reflexion we made around cour process. Thanks to Pr. Sigoillot from the INRA (The French National Institute of Agronomy) we have been able to carry out a relevant process engineering study around application our process could permit. [[1]]
For the detailed interview with Pr.Sigoillot see here.
Our Process is applicable on many ways
Our process concentrate metals, especially platinum and transform it into a highly valuable form, nanoparticles.
To obtain this valuable product, our process begin with raw, worthless, and plentiful materials that can be found almost anywhere since the accumulation due to the catalyst converter occurs everywhere there is a car traffic (urban and road areas). The substrates can be harvest directly in the environment, as some of them presents impressive concentrations like the road dust or the air borne dust. However,it would be much more relevant to integrate our process at the end of an already existing process of treatment, to plug it to the actual network of process. Moreover, solutions of phytoremediation provide a lot of substrates and by-products our project could start from. As most of our process occurs in a controlled environment almost any substrate could be convenient to enter in our process. We could start our process just after the incineration of phytoremedial plants or on the digestat produced by a methanisation
If the raw materials change (substrate), only the first step of our project would change if the basic matter change. The advantage of our process is its easiness of integration into current treatment process. Set up our process would not need a lot of changes in actual facilities.
In this part we ll'detail an industrialized process of what we plan to test in lab condition. We are thankfull to Mr.Sigoillot, who advised us wisely in the choice of which process could be more developed in particular, see the interview here. We'll focus on a process started from sewage sludge. Indeed, choosing sewage sludge as a source of platinum bring another benefit: it will remove platinum from sludges. Nowadays, sludge are so concentrated in metals that it can't be spread on field for valorisation and as a result, sludge are often burnt and ashes are kept in specialized confinement centers, thus making the treatment very expensive and not sustainable. So our process can remove metals aiming to recover it and doing so our process will also achieve the purpose to rid metals from sludges. Moreover, sewage sludge treatment is a payed service by institutions who needs to get rid of it (municipality, motorway operating...) so with this source of platinum our process will start with an already positive financial balance! Of courses polluting metals, preventing spreading in field are many and our process is currently designed to recover specifically and only platinum. But our process is extremely versatile and each step of our process can be modified to be specific of another metal. METTRE LA VIDEO CORRESPONDSANTE ICI
As you will discover in the process, step 4 involves a siderophore that could be specific to another metal. Likewise, the biosorption occurred in the step 9 involves small metals catching peptids, that can be used to catch far more other metals that just only platinum.
During all the explanation of the process, examples displayed in italic are considered for the recovery of 1g of pure platinum.
The Source
Sewage sludge are obtained daily in high amounts, all over the world, as effluents treatment is obviously a continued process. Indeed this source is abondant and inexhaustible, as in almost every city around the world, effluents are treated and sewage sludges are collected. In most of the cases, when metals concentrations in sludges are too high to perform a valorization in the environnement as spreading on fields, the procedure is to stock sludges in a confined place. To reduce the stock volume, sludges are burnt.This step is precisely where our process could be connected to the effluents treatment process network. in some case, the incineration may not be realized yet, so our process should include a incineration step.
If we want to harvest 1g of initial platinum, 1161kg to 3676 kg of sewage sludge ashes should be necessary( see Raw Calculations).
In this first step sludges are simply collected from the effluents treatment network and eventually incinerated thus the product here is ashes.
Bioleaching
The Bioleaching process allow a far better recovery of platinum. Indeed, the drop of pH is required for the metals solubilization. This step could be realized with chemicals as chlorhydric acid in the actual industry. But in order to achieve a greener process as possible, we decided to lower the pH with biological ways, as the leaching accomplished by Thiobacillus. This method is widely use in mines, e.g. for copper mines in South America, so this will be a reliable step already tested in industrial conditions. This method rely on the ability of the bacteria Thiobacilus to acidify its medium until a pH of 1. The bacterium solution is applied on the ashes and liquid part dropping from it constitute the leachate i.e. a very acidified solutions containing solubilized metals particles mostly in ionic form. With this process we hope reach the same leach yield as obtained if realized with Chlorhydric acid i.e. 30% of leached platinum[1].
This step wouldn't be obligatory in our process, but it could improve our recovery yield while still using a environmentally friendly approach. Moreover, after leaching, the remained ashes will present a decreased concentration in metals. If this concentrations are lowered enough, the ashes are now ready to be spreaded on fields. Of courses before it, steps of alcanization and destruction of potentially Thiobacillus cells should be performed. If concentrations are still too high for spreading, this step may be repeated.
This step consist in spreading a Thiobacillus solution on the ashes then recollecting the produced leachate. This step could be also realised not with Thiobacillus but with chemicals solutions.
Siderophore mediated leaching
Even if almost 30 % of platinum has been leached by the drop of pH we need to improve the proportion of leached platinum. As we worked with synthetic biology, we decided to use what it is commonly employed by cells to catch metals, i.e. siderophore. Siderophores are well known to catch iron most of all but some of them have an affinity with others metals as platinum. So in our process, we planned to work with such a siderophore, called Desferrioxamine B. This one is already employed to leach platinum in mines, and have shown high capacity to leach platinum from ores [2].
Leaching yields with DFHOB can reach 78% of the total platinum if both of these conditions are fully respected: a lowering pH level leaching step should be performed, as well as a alcanization of medium (until a pH range between 8 and 9) just before addition of siderophore. The last step devoted to rise up the pH level will be realized using a standard buffer, e.g. a Tris buffer.
So where is the innovation in this step? Firstly, in our case, DFHOB won't be applied on the same materials where is commonly used, in our cases not ores but a leachate of ashes. Basically the main difference will be the metal concentration.
Secondly, DFHOB is usually synthesized chemically, we'll rather produce it in high amounts with bacteria. Indeed, operon of the Desferrioxamine B biosynthesis from Streptomyces coelicolor will be cloned into a E. coli bacteria strains in order to produce it, hence lowering the costs of required basic matter as production by bac