Products
After listening to professor Wu, a member of Chinese Academy Science, we were interested in his research team’s project about MSCs, which was one of the MSCs therapy that had passed the phase IV clinical trail in China. Therefore, we came up with an idea to make a product analysis including market analysis,cost analysis, intellectual property issues, ethics, product safety as well as laws and regulations. Considering MSCs therapy is used to treat various of diseases, which made our analysis become too difficult, we here took severe Crohn’s disease (CD) as an example. Let us show you!
1.Market Analysis
(1) Treatments of severe Crohn’s disease on the market.
Biologic therapies, such as TNF inhibitors, can be used for people with moderately to severely active disease who haven’t responded well to conventional therapy. Remicade (infliximab) and Humira (adalimumab) are the typical drugs.
The price of Remicade and Humira varies based on the dosage, purchasing ways, quality of insurance and many other factors. It’s almost impossible to give a specific price because of these factors.
Besides, MSC therapy has been proved to be effective when other treatments including TNF inhibitors failed. Cupistem© is the first authorized stem cell procedure to treat Crohn's disease in the world.
The following sheet showed comparison among TNF inhibitors and MSCs therapy on the market. (The price information comes from http://www.truemedcost.com/.)

(http://www.truemedcost.com/humira-price/) (CVS Pharmacy)
(2)MSC therapeutics products on the market
At the current market, the price of MSC products varies from $3000 to $40000 per dose, still quite expensive for common patients in therapy. The whole therapy may cost as high as $20000, yet still depending on severity of disease. The decisive factors of the prices include location and sources of MSCs products. Bone marrow is still the most popular source of MSCs.
(The Table below shows various MSCs therapeutics products on the market until 2012.)
 (Reference: Chen, Y. (2016). Mesenchymal Stem Cell: Considerations for Manufacturing and Clinical Trials on Cell Therapy Product. International Journal of Stem Cell Research & Therapy.3:029)
(Reference: Chen, Y. (2016). Mesenchymal Stem Cell: Considerations for Manufacturing and Clinical Trials on Cell Therapy Product. International Journal of Stem Cell Research & Therapy.3:029)
(3)Cost analysis of our project
During our whole experiments, we analyzed the costs and materials at every section and managed to reduce the total cost to a reasonable level.
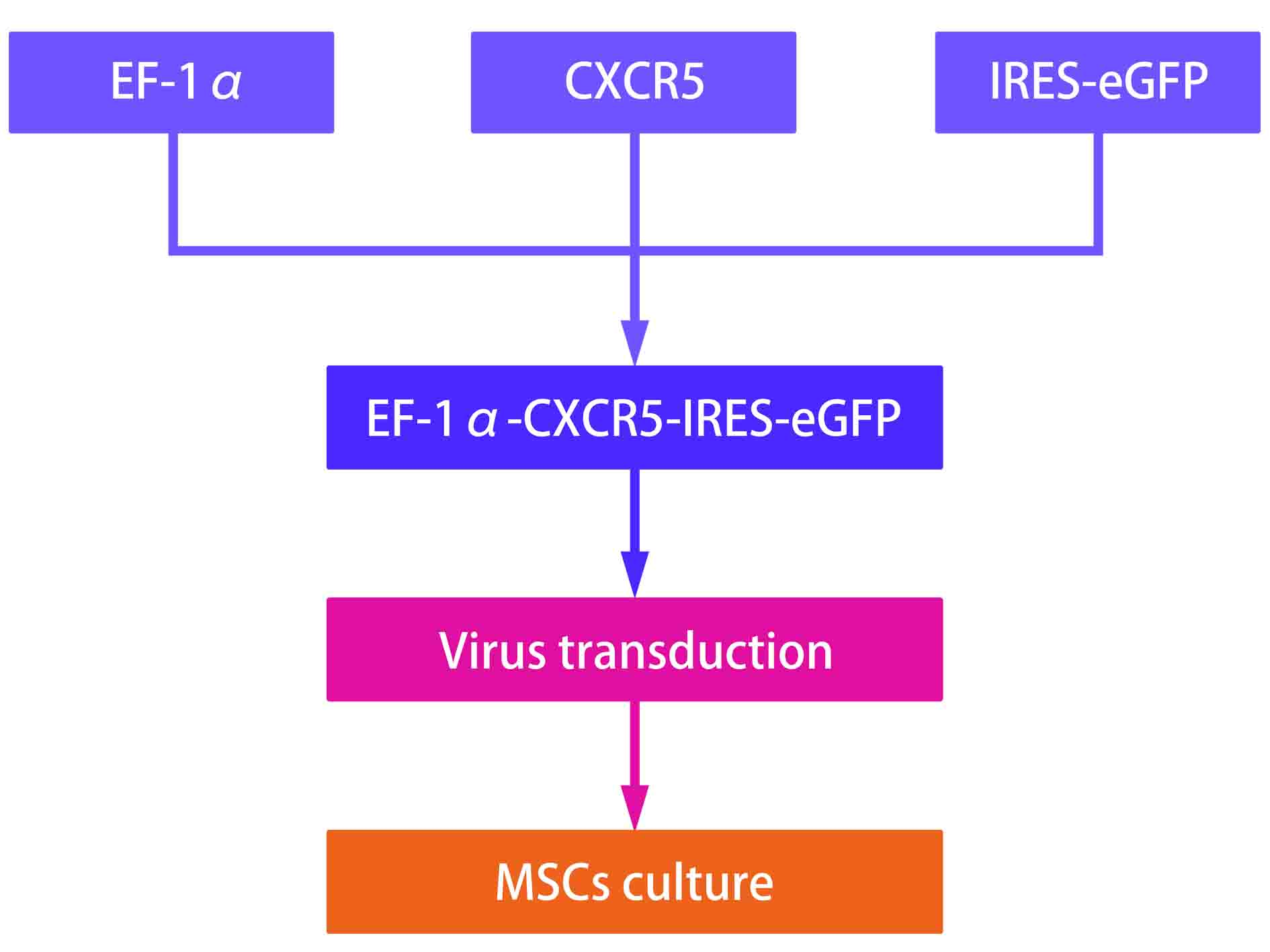






(4)MSCs related clinical trial worldwide
The table below shows there are 3 undergoing clinical trials treating the Crohn’s disease. These three projects may be the potential competitors in the future. For example, Osiris Therapeutics is developing Prochymal (remestemcel-L), treated with gene engineering, for the treatment of moderate to severe CD. Therefore, further improving the safety and efficiency of our project must be our next mission.
 (Reference: Chen, Y. (2016). Mesenchymal Stem Cell: Considerations for Manufacturing and Clinical Trials on Cell Therapy Product. International Journal of Stem Cell Research & Therapy.3:029)
(Reference: Chen, Y. (2016). Mesenchymal Stem Cell: Considerations for Manufacturing and Clinical Trials on Cell Therapy Product. International Journal of Stem Cell Research & Therapy.3:029)
2.Intellectual Property Issues
Undoubtedly, for the benefits of the patients, the intellectual property issues should not be forgotten when we transform our experimental results into products. Therefore, we did a brief analysis of the intellectual property laws worldwide.
By searching the World Intellectual Property database, we figured out which parts of our product have been applied or are originally created by us. According to our analysis results, we drew up our strategy in correspond to the market.
Referring to the World Intellectual Property Organization’s defination of intellectual property rights, we focused on the invention patent section. Invention patent include plant patent, utility patent and design patent.To see more details of the concepts, here are the website.
(http://www.uspto.gov/web/offices/ac/ido/oeip/taf/patdesc.htm)
With some essential knowledge of intellectual property, we then tried to understand the current market and find out the problems of intellectual property we had to face if we transformed our experiment results to products, including what we used belongs to someone else's patents and which parts were originally created by our group and needed an application for new patents.
We used Google Patents (www.google.com.hk/patents/) to do patent search for two aspects: MSCs homing and suicide switch.
The main contents of the two aspects were summarized as follows:
(1) Homing
Keywords
Genetically modification on stem cells even MSCs.
Therapy with MSCs homing to injuries.
Enhancing the homing effects of MSCs by up-regulating chemokine receptors and chemokines gene expression.
Results
Some essential ideas such as genetically engineered MSCs (CA 2567177 A1), the use of MSC immunomodulatory function in therapy (WO 2002156522 A1), the use of chemokine and chemokine receptor in enhancing MSC homing effect(US 20070020230 A1) (CA 2447167 A1 ) have already been patented by others.
Our project is to enhance the homing effect of MSCs by up-regulating chemokine receptors gene expression. The use of CXC family (except CXCR4) and CX3C family. However, part of the CCR family such as CCR 1,CCR2, CCR3, CCR5 were clearly applied for patents (US 8445453 B2) and CCR3,CCR6,CCR8 were applied as targets to induce stem cells homing. (WO2004084931 A1).
Therefore, our application can focus on the chemokines and corresponding receptors which are not mentioned above.
(2)Suicide switch
Keywords
The marker of MSCs fibrosis process--alpha-SMA.
Suicide gene
Switch kits.
Results
It has been prompted that MSCs suicide genes express thymidine enzymes to kill cancer cells. Some patents mentioned that low expression of α-SMA is an isolation marker of MSCs (CN 101272798 B), but they did not indicate that α-SMA as a criterion for testing MSC fibrosis. Patents indicating promoter of α-SMA as a suicide switch are also not found.
Therefore, utilizing the promoter of α-SMA in MSC as a suicide gene switch ensured as novel ideas and should be patented.
What should we do next?
Firstly, patent our novel ideas. Secondly, connect with the owners of related patents.
(1)Patent applying steps

(2)The license of patent can be negotiated.
The related regulations about the negotiation in China are as followed:
1.Any assignment, by a Chinese entity or individual, of the right to apply for a patent, or of the patent right, to a foreigner must be approved by the competent department concerned of the State Council.
2.Where the right to apply for a patent or the patent right is assigned, the parties shall conclude a written contract and register it with the patent administration department under the State Council. The patent administration department under the State Council shall announce the registration.
3.Any entity or individual exploiting the patent of another shall conclude with the patentee a written license contract for exploitation and pay the patentee a fee for the exploitation of the patent.
Eventually, if our product needs others patents’ licenses, we will negotiate with the owners for win-win cooperation, even buy their patent licenses.
At this point, our team had completed human practice part related to the intellectual property issues and predicted the problems likely to be encountered and discussed the appropriate solutions.
3.Laws and regulations
Till now, no MSCs therapeutics product is approved in China. Hence, investigating Chinese government’s movement on cell therapy is of great importance. Click here to see what we gain through our work.
4.product safety
Product safety is always an essential aspect. Click here to find out what we investigated.
5.Ethics
Moreover, future MSC therapeutics product also involves ethic issues. Click here to see the details.

 iGEMxSYSU-MEDICINE
iGEMxSYSU-MEDICINE
 IGEM SYSU-Medicine
IGEM SYSU-Medicine
 SYSU_MEDICINE@163.com
SYSU_MEDICINE@163.com
 SYSU-MEDICINE
SYSU-MEDICINE
 iGEM SYSU-MEDICINE
iGEM SYSU-MEDICINE





