| Line 101: | Line 101: | ||
</center> | </center> | ||
| − | <p> Isolated colonies were selected from each "isolation plate" and continually grown up and streaked out to ensure that the resulting frozen stock was truly axenic. Each newly isolated microbe was designated with a "KOM #" based on the order in which it was isolated (i.e. KOM 01, KOM 02, etc.) to serve as a placeholder name until the species could be identified. In order to begin this identification process, genomic DNA (gDNA) was first isolated from each individual strain. This DNA was then used as the template for two separate PCR reactions targeting either the 16S rRNA gene in bacteria, or the ITS rRNA gene for fungi. PCR products were then run on a 1% agarose gel to observe which reaction yielded product in gel. | + | <p> Isolated colonies were selected from each "isolation plate" (<b>Figure 1</b>) and continually grown up and streaked out to ensure that the resulting frozen stock was truly axenic. Each newly isolated microbe was designated with a "KOM #" based on the order in which it was isolated (i.e. KOM 01, KOM 02, etc.) to serve as a placeholder name until the species could be identified. In order to begin this identification process, genomic DNA (gDNA) was first isolated from each individual strain. This DNA was then used as the template for two separate PCR reactions targeting either the 16S rRNA gene in bacteria, or the ITS rRNA gene for fungi. PCR products were then run on a 1% agarose gel to observe which reaction yielded product in gel (<b>Figure 2</b>). |
</p> | </p> | ||
<p> | <p> | ||
| − | Once it was determined whether each isolate was a bacterium or a fungus, the PCR products were purified and samples of the gDNA was sequenced using Sanger sequencing. The resulting sequences were then run through the Ribosomal Database Project (RDP) SeqMatch tool in order to identify the exact species of bacteria or yeast that correspond to each tested isolate. The identified microbes are listed below in Table 1. | + | Once it was determined whether each isolate was a bacterium or a fungus, the PCR products were purified and samples of the gDNA was sequenced using Sanger sequencing. The resulting sequences were then run through the Ribosomal Database Project (RDP) SeqMatch tool in order to identify the exact species of bacteria or yeast that correspond to each tested isolate. The identified microbes are listed below in <b>Table 1</b>. |
<br> | <br> | ||
</p> | </p> | ||
| Line 183: | Line 183: | ||
<p>We have attempted to conjugate GFP into both <i>G. oxydans</i> and <i>G. hansenii</i> with a Diaminopimelic Acid (DAP) auxotrophic strain of <i> E. coli</i> (The Barrick Lab). The plasmid contains the vector pMMB67EH, the promoter PA-1, GFP and a spectinomycin resistance gene. </p> | <p>We have attempted to conjugate GFP into both <i>G. oxydans</i> and <i>G. hansenii</i> with a Diaminopimelic Acid (DAP) auxotrophic strain of <i> E. coli</i> (The Barrick Lab). The plasmid contains the vector pMMB67EH, the promoter PA-1, GFP and a spectinomycin resistance gene. </p> | ||
| − | <p>The first conjugation was done with two of our isolated <i>G. oxydans</i> strains, in case the strains might behave differently. First, a mixture between our recipient strain and the DAP auxotroph strain were plated on an LB+DAP agar plate to allow for conjugation to occur. After 24 hours of incubation, we scraped up the growth and plated each conjugation mixture onto a LB+Spec plate. 24-48 hours later, we viewed the potential transconjugants using a fluorescence microscope. We then picked these glowing colonies and streaked them out onto another LB+Spec plate. We then followed our protocol for genome DNA isolation and 16S sequencing, as described above, to confirm successful conjugation of <i>G. oxydans</i>. After troubleshooting our 16s procedure, we were finally able to obtain a viable sequencing result. However, all of the glowing colonies were identified as <i>E. coli</i>.</p> | + | <p>The first conjugation was done with two of our isolated <i>G. oxydans</i> strains, in case the strains might behave differently. First, a mixture between our recipient strain and the DAP auxotroph strain were plated on an LB+DAP agar plate to allow for conjugation to occur. After 24 hours of incubation, we scraped up the growth and plated each conjugation mixture onto a LB+Spec plate (<b>Figure 1</b>). 24-48 hours later, we viewed the potential transconjugants using a fluorescence microscope. We then picked these glowing colonies and streaked them out onto another LB+Spec plate. We then followed our protocol for genome DNA isolation and 16S sequencing, as described above, to confirm successful conjugation of <i>G. oxydans</i>. After troubleshooting our 16s procedure, we were finally able to obtain a viable sequencing result. However, all of the glowing colonies were identified as <i>E. coli</i>.</p> |
<div class="floatleft"> | <div class="floatleft"> | ||
| Line 198: | Line 198: | ||
<p>We then decided to perform a Minimum Inhibitory Concentration (MIC) experiment in order to determine if <i>G. oxydans</i> is able to survive antibiotics above the standard <i>E. coli</i> concentration. We tested the antibiotics kanamycin, spectinomycin and carbenicillin.</p> | <p>We then decided to perform a Minimum Inhibitory Concentration (MIC) experiment in order to determine if <i>G. oxydans</i> is able to survive antibiotics above the standard <i>E. coli</i> concentration. We tested the antibiotics kanamycin, spectinomycin and carbenicillin.</p> | ||
| − | <p>Our results showed that <i>G. oxydans</i> is resistant to at least 4x concentrations of spectinomycin (1x = 60µg/mL) and carbenicillin (1x = 100µg/mL). However, a 1x concentration of kanamycin (1x = 50µg/mL) was sufficient to inhibit growth. With this information, we then performed conjugations with <i>E. coli</i> donors that had a kanamycin resistance. These results are still pending through the wiki freeze. | + | <p>Our results in <b> Figure 2</b> showed that <i>G. oxydans</i> is resistant to at least 4x concentrations of spectinomycin (1x = 60µg/mL) and carbenicillin (1x = 100µg/mL). However, a 1x concentration of kanamycin (1x = 50µg/mL) was sufficient to inhibit growth. With this information, we then performed conjugations with <i>E. coli</i> donors that had a kanamycin resistance. These results are still pending through the wiki freeze. |
<center> | <center> | ||
| Line 308: | Line 308: | ||
</html> | </html> | ||
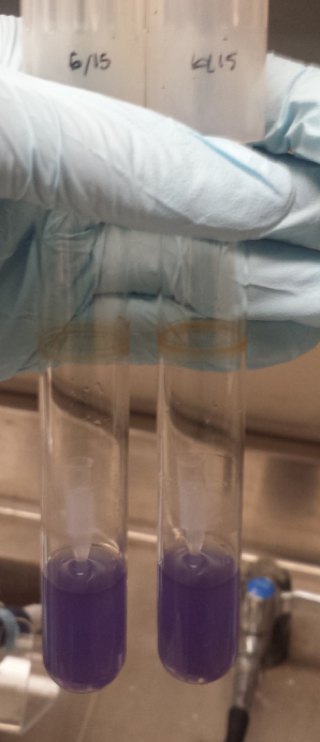
| − | [[File:T--Austin_UTexas--Cpx_pH_Culture_Tubes_2.png|thumb|right|549px| Figure 1. Testing the CpxR Construct in pH 6-9. From left to right is control pH 6-9 and then experimental pH 6-9. These are showing the gradient change in expression accordingly with the change of pH due to a pH-dependent promotor compared to consistent expression accordingly with a promoter that is always "on". Credit: Sofia Chinea]] | + | [[File:T--Austin_UTexas--Cpx_pH_Culture_Tubes_2.png|thumb|right|549px| <b>Figure 1</b>. Testing the CpxR Construct in pH 6-9. From left to right is control pH 6-9 and then experimental pH 6-9. These are showing the gradient change in expression accordingly with the change of pH due to a pH-dependent promotor compared to consistent expression accordingly with a promoter that is always "on". Credit: Sofia Chinea]] |
<html> | <html> | ||
<p>CpxA-CpxR is a two-component mechanism that is activated at pH 7.4 and repressed at pH 6.0. CpxA is an intermembrane protein that autophosphorylates at a certain external pH, CpxR (a kinase) then gets phosphorylated by CpxA and acts as a transcription factor. This system originally is a transcription factor for the virF gene, but virF was replaced with a reporter. The original sequence was found in <i>Shigella sonnei</i>, but <i>E. coli</i> has a homolog of these proteins so all that is required on the construct is the appropriate prefix/suffix and CpxR binding site (Nakayama and Watanabe, 1995; Nakayama and Watanabe, 1998). | <p>CpxA-CpxR is a two-component mechanism that is activated at pH 7.4 and repressed at pH 6.0. CpxA is an intermembrane protein that autophosphorylates at a certain external pH, CpxR (a kinase) then gets phosphorylated by CpxA and acts as a transcription factor. This system originally is a transcription factor for the virF gene, but virF was replaced with a reporter. The original sequence was found in <i>Shigella sonnei</i>, but <i>E. coli</i> has a homolog of these proteins so all that is required on the construct is the appropriate prefix/suffix and CpxR binding site (Nakayama and Watanabe, 1995; Nakayama and Watanabe, 1998). | ||
</html> | </html> | ||
| − | [[File:T--Austin_UTexas--YGCPtube.png|thumb|left|275px|Figure | + | [[File:T--Austin_UTexas--YGCPtube.png|thumb|left|275px|<b>Figure 2</b>. amajLime expressed in <i>E. coli</i> in liquid LB. Credit: Sofia Chinea]] |
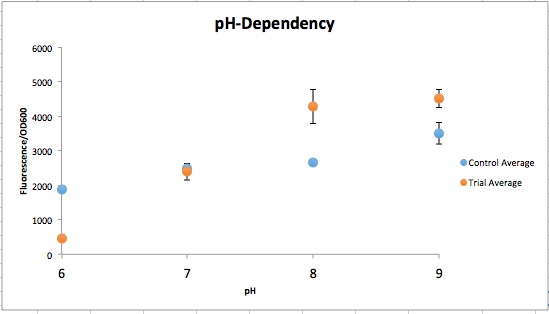
| − | [[File:T--Austin_UTexas--pH_Dependent_Promoter.jpeg|thumb|right|549px| Figure | + | [[File:T--Austin_UTexas--pH_Dependent_Promoter.jpeg|thumb|right|549px| <b>Figure 3</b>. Normalized fluorescent values from CpxR construct vs control (YGCP). The fluorescence per cell count stayed generally the same throughout the range of pH while the CpxR has a clear increase in fluorescence per cell. Credit: Sofia Chinea]]<html> |
| − | <p>The order from left to right in figure 1 is control pH 6-9 and then Experimental pH 6-9. These are showing the gradient change in expression accordingly with the change of pH due to a pH-dependent promotor compared to consistent expression accordingly with a promoter that is always "on". The main point is that the control at pH 6 has more expression of the yellow-green chromoprotein than the Experimental at pH 6. The pH-dependent promoter of the experimental group is down-regulated at pH 6 whereas the control is not. Also, there is an increase in YGCP expression between the experiment pH 7 and pH 8 that is not seen in the control between pH 7 and pH 8. The normalized data in figure | + | <p>The order from left to right in <b>figure 1</b> is control pH 6-9 and then Experimental pH 6-9. These are showing the gradient change in expression accordingly with the change of pH due to a pH-dependent promotor compared to consistent expression accordingly with a promoter that is always "on". The main point is that the control at pH 6 has more expression of the yellow-green chromoprotein than the Experimental at pH 6. The pH-dependent promoter of the experimental group is down-regulated at pH 6 whereas the control is not. Also, there is an increase in YGCP expression between the experiment pH 7 and pH 8 that is not seen in the control between pH 7 and pH 8. The normalized data in <b>figure 3</b> shows the relative expression of YGCP. The CpxA-CpxR construct can be found on the iGEM registry as: <a href=“http://parts.igem.org/Part:Bba_K2097000”>BBa_K2097000</a>, while the construct utilized as a control can be found on the iGEM registry as <a href="http://parts.igem.org/Part:BBa_2097002">BBa_K2097002</a> as well as in <b>figure 2</b>.</p> |
<br> | <br> | ||
| Line 325: | Line 325: | ||
<h4>P-atp2</h4> | <h4>P-atp2</h4> | ||
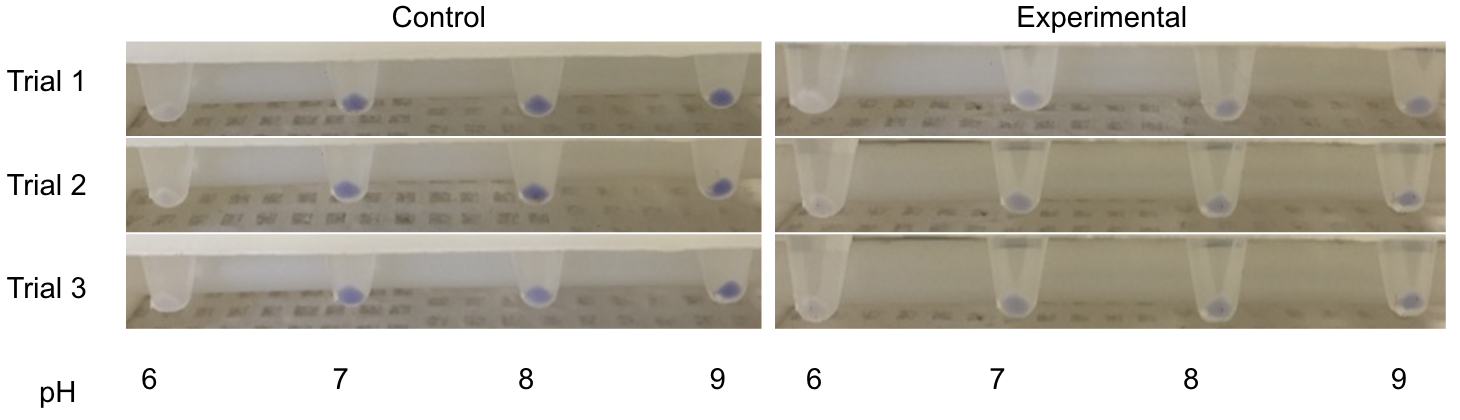
| − | <p>The P-atp2 promoter, native to the bacterium <i>Corynebacterium glutamicum</i> is reportedly induced at pH 7, to pH 9 (<a href="https://2015.igem.org/Team:BIT-China/Parts">BIT-China-2015</a> and <a href="http://parts.igem.org/Part:BBa_K1675021">BBa_K1675021</a>). Utilizing the blue chromoprotein (<a href="http://partsregistry.org/Part:BBa_K592009">BBa_K592009</a>), a test was designed in which a plasmid containing the P-atp2 promoter with the blue chromoprotein was grown alongside an <i>E. coli</i> line that contained a plasmid with just the blue chromoprotein. We expected to see constant blue chromoprotein production in the control series (those that lacked P-atp2) and a visual increase in blue chromoprotein as the pH was raised from 6 to 9 in the cells that contained the P-atp2 construct. The construct utilized as a control can be found on the iGEM registry <a href="http://parts.igem.org/Part:BBa_2097001">BBa_K2097001</a> as as in figure | + | <p>The P-atp2 promoter, native to the bacterium <i>Corynebacterium glutamicum</i> is reportedly induced at pH 7, to pH 9 (<a href="https://2015.igem.org/Team:BIT-China/Parts">BIT-China-2015</a> and <a href="http://parts.igem.org/Part:BBa_K1675021">BBa_K1675021</a>). Utilizing the blue chromoprotein (<a href="http://partsregistry.org/Part:BBa_K592009">BBa_K592009</a>), a test was designed in which a plasmid containing the P-atp2 promoter with the blue chromoprotein was grown alongside an <i>E. coli</i> line that contained a plasmid with just the blue chromoprotein. We expected to see constant blue chromoprotein production in the control series (those that lacked P-atp2) and a visual increase in blue chromoprotein as the pH was raised from 6 to 9 in the cells that contained the P-atp2 construct. The construct utilized as a control can be found on the iGEM registry <a href="http://parts.igem.org/Part:BBa_2097001">BBa_K2097001</a> as as in <b>figure 4</b>.</p> |
| − | <p>However, as seen in figure | + | <p>However, as seen in <b>figure 5</b>, no clear change in color expression appears in the experimental trials, suggesting a lack of sensitivity of the P-atp2 promoter.</p> |
</html> | </html> | ||
| − | [[File:T--Austin_UTexas--BCPtube.png|thumb|left|150px| Figure | + | [[File:T--Austin_UTexas--BCPtube.png|thumb|left|150px| <b>Figure 4</b>. amilCP expressed in <i> E. coli </i> and in liquid LB. Credit: Riya Sreenivasan]] |
| − | [[File:T--Austin_UTexas--Patp2Results.png|thumb|right|600px| Figure | + | [[File:T--Austin_UTexas--Patp2Results.png|thumb|right|600px| <b>Figure 5</b>. Spun down P-atp2 constructs compared to controls in pH6-9. There is no clear gradient change in color expression. Credit: Ian Overman and Alex Alario]] |
<html> | <html> | ||
</div> | </div> | ||
Revision as of 00:34, 20 October 2016
Results

Click on one of the images below to learn more about our results!
GOX Sequences as Putative Promoters
Three endogenous upstream regions of loci on the Gluconobacter oxydans chromosome were reported to show increased mRNA synthesis as pH decreased, were isolated and obtained, as seen in table 1 (Hanke, et al., 2012). Using Golden Gate assembly, these putative promoters have been placed on the Golden Gate entry vector pYTK001 for later use. By utilizing these pH-sensitive promoters with different reporters and transforming them into multiple organisms in kombucha, the visualization of the microbes and their location in kombucha would be possible (Lee, et al., 2015). This will serve as a stepping stone into further understanding how the microbiome of kombucha changes as it brews as well as determining organism concentration specific times during the brewing process.
| Locus Tag | Predicted Functions | mRNA ratio pH4/pH6 |
|---|---|---|
| GOX0647 | Putative exporter protein, ArAE family | 12.91 |
| GOX0890 | Hypothetical protein GOX0890 | 4.93 |
| GOX1841 | Hypothetical protein GOX1841 | 3.36 |
References
- The Barrick Lab Conjugation Protocol
- Abbot, J. Komagataeibacter xylinus isolate ATCC53582 genome assembly, contig: ATCC53582_Chromosome, whole genome shotgun sequence. 2015. Accessed from NCBI website.
- BIT-China-2015
- Calloway, Ewen. (2015) Lab staple agar hit by seaweed shortage. Nature.
- Hanke, T., Richhardt, J., Polen, T., Sahm, H., Bringer, S., and Bott, M. (2012) Influence of oxygen limitation, absence of the cytochrome bc1 complex and low pH on global gene expression in Gluconobacter oxydans 621H using DNA microarray technology. Journal of Biotechnology 157, 359–372.
- Ioannis Giavasis et al. (2000) Gellan Gum Critical Reviews in Biotechnology., 20.3: 177-211
- Kang, Kenneth S. et al. (1982) Agar-Like Polysaccharide Produced by a Pseudomonas Species: Production and Basic Properties. Applied and Environmental Microbiology., 1086-1091
- Kuper, C., and Jung, K. (2005) CadC-mediated activation of the cadBA promoter in Escherichia coli. Journal of Molecular and Microbiological Biotechnology 1, 26–39.
- Lee ME, DeLoache, WC A, Cervantes B, Dueber, JE. (2015) A Highly-characterized Yeast Toolkit for Modular, Multi-part Assembly. ACS Synthetic Biology 4 975-986
- Mamlouk, Y. and M. Gullo. Acetic Acid Bacteria: Physiology and carbon sources oxidation. 2013. Indian Journal of Microbiology 53 (4): 337-384.
- Nakayama, S.-I., and Watanabe, H. (1998) Identification of cpxR as a Positive Regulator Essential for Expression of the Shigella sonnei virF Gene. Journal of Bacteriology 180, 3522–3528.
- Nakayama, S.-I., and Watanabe, H. (1995) Involvement of cpxA, a Sensor of a Two-Component Regulatory System, in the pH-Dependent Regulation of Expression of Shigella sonnei virF Gene. Journal of Bacteriology 177, 5062–5069.
- Robillard, R. A microbial breathalyzer: design of a colorimetric assay for the detection and quantification of ethanol production in microbes. 2007. Major qualifying project for a B.S. degree from Worcester Polytechnic Institute.
- Wang, Xia, et al. (2006) Modeling for Gellan Gum Production by Sphingomonas paucimobilis ATCC 31461 in a Simplified Medium. Applied and Environmental Microbiology, 3367-3374
- Wu et. al. (2014) Yellow pigments generation deficient Sphingomonas strain and application thereof in Gellan Gum. US Patent 8,685,698.
Back to Top