| Line 24: | Line 24: | ||
} | } | ||
| − | .agarinfo, .electrophoresisinfo, .glucoseinfo, .glycerolinfo, .mediuminfo{ | + | .agarinfo, .electrophoresisinfo, .glucoseinfo, .glycerolinfo, .mediuminfo, .SOBmediuminfo{ |
display:none; | display:none; | ||
border: solid 1px black; | border: solid 1px black; | ||
| Line 239: | Line 239: | ||
<button class="SOBmedium sub_sub_title">SOB medium (Super Optimal Broth)</button> | <button class="SOBmedium sub_sub_title">SOB medium (Super Optimal Broth)</button> | ||
| + | <div class="SOBmediuminfo"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/5/5e/T--Manchester--protocoltable7.png" alt="table 7" /> | ||
| + | |||
| + | <ol> | ||
| + | <li>Prepare the solution by mixing the ingredients stated above. </li> | ||
| + | <li>Sterilize in an autoclave before using it to prepare SOC medium.</li> | ||
| + | </ol> | ||
| + | <br /> | ||
| − | </div> | + | <p><i>Additional notes</i></p> |
| + | <ul> | ||
| + | <li><i>Some formulations of SOB use 10 mM MgCl<sub>2</sub> and 10 mM MgSO<sub>4</sub> instead of 20mM MgSO<sub>4</sub>. </i></li> | ||
| + | <li><i>SOB medium is also available dry pre-mixed from Difco, 0443-17</i></li> | ||
| + | <li><i>Adjust to pH 7.5 prior to use. This requires approximately 25mL of 1M NaOH per liter. </i></li> | ||
| + | </ul> | ||
| + | <br /> | ||
| + | <p>Source from <a href="http://openwetware.org/wiki/SOB" target="_blank">OpenWetWare</a></p> | ||
| + | |||
| + | </div> | ||
</div> | </div> | ||
| Line 275: | Line 292: | ||
$(".medium").click(function(){ | $(".medium").click(function(){ | ||
$(".mediuminfo").toggle(); | $(".mediuminfo").toggle(); | ||
| + | }); | ||
| + | }); | ||
| + | |||
| + | $(document).ready(function(){ | ||
| + | $(".SOBmedium").click(function(){ | ||
| + | $(".SOBmediuminfo").toggle(); | ||
}); | }); | ||
}); | }); | ||
Revision as of 09:28, 24 September 2016


Medium and Buffers

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare the SOC medium.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.
*Source from 2015 iGEM Exeter
For nucleic acid DNA/RNA separation
- TAE buffer (Tris-acetate-EDTA)
- TBE buffer (Tris-borate-EDTA)
- LB buffer (Lithium borate)

- Prepare the solution by mixing the ingredients stated above.
If not using pre-mixed LB agar powder, prepare the materials as below:
- In a 1L Erlenmeyer flask, swirl and mix the solution.
- Cover the top of the flask with a lid/aluminum foil and label with autoclave tape.
- Autoclave the liquid setting for 20 minutes or according to your autoclave's specifications.
- After removing the solution from the autoclave, allow the agar solution to cool to 55°C in an oven or water bath.
- When pouring the LB agar into plates, keep the bench area sterile by working near a flame or Bunsen burner. Alternatively, prepare the plates in a vacuum hood.
- Add the appropriate amount of desired antibiotic (refer the table below) to the solution and swirl to mix.
- Pour approximately 20mL of LB agar per 10cm polystyrene Petri dish.
- Place the lids on the plates and allow them to cool for until the agar is solidified.
- Label the bottom of plates with antibiotic and date before storing in plastic bags or sealed with Parafilm at 4°C.

Table source from New England Biolabs
Additional note:
- Antibiotic carbenicillin can be substituted for ampicillin in antibiotic selection plates [1].
If not using pre-mixed LB broth powder, prepare the materials as below:

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.

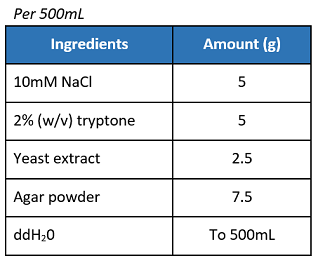
- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare SOC medium.
Additional notes
- Some formulations of SOB use 10 mM MgCl2 and 10 mM MgSO4 instead of 20mM MgSO4.
- SOB medium is also available dry pre-mixed from Difco, 0443-17
- Adjust to pH 7.5 prior to use. This requires approximately 25mL of 1M NaOH per liter.
Source from OpenWetWare
















