| Line 24: | Line 24: | ||
} | } | ||
| − | .agarinfo, .electrophoresisinfo, .glucoseinfo, .glycerolinfo, .mediuminfo, .SOBmediuminfo, .SOCmediuminfo{ | + | .agarinfo, .electrophoresisinfo, .glucoseinfo, .glycerolinfo, .mediuminfo, .SOBmediuminfo, .SOCmediuminfo, .TSSbufferinfo{ |
display:none; | display:none; | ||
border: solid 1px black; | border: solid 1px black; | ||
| Line 117: | Line 117: | ||
<br /> | <br /> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<div class="glucoseinfo"> | <div class="glucoseinfo"> | ||
<img src="https://static.igem.org/mediawiki/2016/2/2f/T--Manchester--protocoltable1.png" alt="table 1" /> | <img src="https://static.igem.org/mediawiki/2016/2/2f/T--Manchester--protocoltable1.png" alt="table 1" /> | ||
| Line 271: | Line 253: | ||
</ol> | </ol> | ||
| − | <p>Source from <a href="http://openwetware.org/wiki/SOC" alt="table 8"> OpenWetWare</a></p> | + | <p>*Source from <a href="http://openwetware.TSS buffer (Transformation & Storage Solution) org/wiki/SOC" alt="table 8"> OpenWetWare</a></p> |
</div> | </div> | ||
| Line 277: | Line 259: | ||
| + | <button class="TSSbuffer">TSS buffer (Transformation & Storage Solution)</button> | ||
| + | <div class="TSSbufferinfo"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/8/82/T--Manchester--protocoltable9.png" alt="table 9"> | ||
| + | <ol> | ||
| + | <li>Prepare the solution by mixing the ingredients stated above.</li> | ||
| + | <li>Filter sterilize using a 0.22 μm filter.</li> | ||
| + | <li>Store at 4°C or -20°C.</li> | ||
| + | </ol> | ||
| + | <br /> | ||
| + | <p>*Source from <a href="http://openwetware.org/wiki/TSS" target="_blank"> OpenWetWare: TSS</a></p> | ||
| + | </div> | ||
| + | |||
| + | <div class="column full_size"> | ||
| + | <div class="divindiv" style="margin:auto"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/b/b4/T--Manchester--protocols_bigtitle.png" alt="symbol" style="width:120px;float:left;" /> | ||
| + | <h1 class="sub_title">Transformation</h1> | ||
| + | </div> | ||
</div> | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | <!-- | ||
| + | <p style="padding-left:10px;padding-bottom:0px"><i>per 1L</i></p> | ||
| + | |||
| + | |||
| + | <table style="border:solid 1px black;border-collapse:collapse;" class="protocols_table"> | ||
| + | <tr> | ||
| + | <th style="border:solid 1px black;background-color:rgb(46,116,181);color: white;padding:10px 40px;">Ingredients</th> | ||
| + | <th style="border:solid 1px black;background-color:rgb(46,116,181);color: white;padding:10px 40px;">Amount</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="border:solid 1px black;padding:10px 40px;">Glucose</td> | ||
| + | <td style="border:solid 1px black;padding:10px 40px;">200g</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="border:solid 1px black;padding:10px 40px;">ddH<sub>2</sub>O</td> | ||
| + | <td style="border:solid 1px black;padding:10px 40px;">To 1L</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | --> | ||
| + | |||
</body> | </body> | ||
| Line 327: | Line 355: | ||
$(".SOCmedium").click(function(){ | $(".SOCmedium").click(function(){ | ||
$(".SOCmediuminfo").toggle(); | $(".SOCmediuminfo").toggle(); | ||
| + | }); | ||
| + | }); | ||
| + | |||
| + | $(document).ready(function(){ | ||
| + | $(".TSSbuffer").click(function(){ | ||
| + | $(".TSSbufferinfo").toggle(); | ||
}); | }); | ||
}); | }); | ||
Revision as of 10:29, 24 September 2016


Medium and Buffers

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare the SOC medium.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.
*Source from 2015 iGEM Exeter
For nucleic acid DNA/RNA separation
- TAE buffer (Tris-acetate-EDTA)
- TBE buffer (Tris-borate-EDTA)
- LB buffer (Lithium borate)

- Prepare the solution by mixing the ingredients stated above.
If not using pre-mixed LB agar powder, prepare the materials as below:
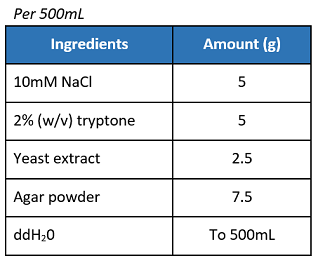
- In a 1L Erlenmeyer flask, swirl and mix the solution.
- Cover the top of the flask with a lid/aluminum foil and label with autoclave tape.
- Autoclave the liquid setting for 20 minutes or according to your autoclave's specifications.
- After removing the solution from the autoclave, allow the agar solution to cool to 55°C in an oven or water bath.
- When pouring the LB agar into plates, keep the bench area sterile by working near a flame or Bunsen burner. Alternatively, prepare the plates in a vacuum hood.
- Add the appropriate amount of desired antibiotic (refer the table below) to the solution and swirl to mix.
- Pour approximately 20mL of LB agar per 10cm polystyrene Petri dish.
- Place the lids on the plates and allow them to cool for until the agar is solidified.
- Label the bottom of plates with antibiotic and date before storing in plastic bags or sealed with Parafilm at 4°C.

Table source from New England Biolabs
Additional note:
- Antibiotic carbenicillin can be substituted for ampicillin in antibiotic selection plates [1].
If not using pre-mixed LB broth powder, prepare the materials as below:

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare SOC medium.
Additional notes
- Some formulations of SOB use 10 mM MgCl2 and 10 mM MgSO4 instead of 20mM MgSO4.
- SOB medium is also available dry pre-mixed from Difco, 0443-17
- Adjust to pH 7.5 prior to use. This requires approximately 25mL of 1M NaOH per liter.
Source from OpenWetWare

- Prepare the solution by mixing the ingredients stated above.
- Filter sterilize using a 0.22 μm filter.
- Store at 4°C or -20°C.
*Source from OpenWetWare: TSS

Transformation

















