| Line 289: | Line 289: | ||
<p><i>Ingredients:</i></p> | <p><i>Ingredients:</i></p> | ||
<ul> | <ul> | ||
| − | <li><i><a href="#medium">LB media</a></i></li> | + | <li><i><a class="LBmedia" href="#medium">LB media</a></i></li> |
| − | <li><i><a href="#glycerol">50% (w/v) glycerol solution</a></i></li> | + | <li><i><a class="gly" href="#glycerol">50% (w/v) glycerol solution</a></i></li> |
</ul> | </ul> | ||
| Line 393: | Line 393: | ||
</script> | </script> | ||
| + | |||
| + | <script> | ||
| + | |||
| + | $(document).ready(function(){ | ||
| + | $(".LBmedia").click(function(){ | ||
| + | $(".mediuminfo").show(); | ||
| + | }); | ||
| + | }); | ||
| + | |||
| + | $(document).ready(function(){ | ||
| + | $(".gly").click(function(){ | ||
| + | $(".glycerolinfo").show(); | ||
| + | }); | ||
| + | }); | ||
</html> | </html> | ||
{{Manchester/CSS/footer}} | {{Manchester/CSS/footer}} | ||
Revision as of 21:35, 24 September 2016


Medium and Buffers

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare the SOC medium.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.
*Source from 2015 iGEM Exeter
For nucleic acids DNA/RNA separation
- TAE buffer (Tris-acetate-EDTA)
- TBE buffer (Tris-borate-EDTA)
- LAB buffer (Lithium-acetate-borate)

- Prepare the solution by mixing the ingredients stated above.
If not using pre-mixed LB agar powder, prepare the materials as below:
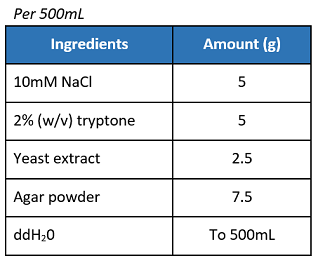
- In a 1L Erlenmeyer flask, swirl and mix the solution.
- Cover the top of the flask with a lid/aluminum foil and label with autoclave tape.
- Autoclave the liquid setting for 20 minutes or according to your autoclave's specifications.
- After removing the solution from the autoclave, allow the agar solution to cool to 55°C in an oven or water bath.
- When pouring the LB agar into plates, keep the bench area sterile by working near a flame or Bunsen burner. Alternatively, prepare the plates in a vacuum hood.
- Add the appropriate amount of desired antibiotic (refer the table below) to the solution and swirl to mix.
- Pour approximately 20mL of LB agar per 10cm polystyrene Petri dish.
- Place the lids on the plates and allow them to cool for until the agar is solidified.
- Label the bottom of plates with antibiotic and date before storing in plastic bags or sealed with Parafilm at 4°C.

Table source from New England Biolabs
Additional note:
- Antibiotic carbenicillin can be substituted for ampicillin in antibiotic selection plates [1].
If not using pre-mixed LB broth powder, prepare the materials as below:

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare SOC medium.
Additional notes
- Some formulations of SOB use 10 mM MgCl2 and 10 mM MgSO4 instead of 20mM MgSO4.
- SOB medium is also available dry pre-mixed from Difco, 0443-17
- Adjust to pH 7.5 prior to use. This requires approximately 25mL of 1M NaOH per liter.
Source from OpenWetWare

- Prepare the solution by mixing the ingredients stated above.
- Filter sterilize using a 0.22 μm filter.
- Store at 4°C or -20°C.
*Source from OpenWetWare: TSS

Transformation
Ingredients:
Before starting the procedure, be sure to:
- Pre-chill centrifuge and cool rotor to 4°C
- Pre-chill 1L of 10% (w/v) glycerol solution on ice
- Pre-chill 500mL/1000mL Falcon tubes on ice

