| Line 163: | Line 163: | ||
</div> | </div> | ||
| − | <button class="agar sub_sub_title">LB agar (Luria-Bertani agar)</button> | + | <button id="agar" class="agar sub_sub_title">LB agar (Luria-Bertani agar)</button> |
<br /> | <br /> | ||
<div class="agarinfo"> | <div class="agarinfo"> | ||
| Line 244: | Line 244: | ||
| − | <button class="SOCmedium sub_sub_title">SOC medium (Super Optimal broth with Catabolite repression)</button> | + | <button id="SOCmedium" class="SOCmedium sub_sub_title">SOC medium (Super Optimal broth with Catabolite repression)</button> |
<div class="SOCmediuminfo"> | <div class="SOCmediuminfo"> | ||
| Line 299: | Line 299: | ||
<li><i>Pre-chill 500mL/1000mL Falcon tubes on ice</i></li> | <li><i>Pre-chill 500mL/1000mL Falcon tubes on ice</i></li> | ||
</ul> | </ul> | ||
| + | |||
| + | <br /> | ||
| + | |||
| + | <p><i>During the procedure: <br />Work under flame at all times.</i></p> | ||
| + | |||
| + | <br /> | ||
| + | |||
| + | <ol> | ||
| + | <li>Grow a 5mL overnight culture of cells in LB media.</li> | ||
| + | <li>In the morning, dilute this culture by at least 1/100 into a 50mL of fresh LB media in a 200mL conical flask.</li> | ||
| + | <li>Incubate them at 37°C. </li> | ||
| + | <li>Monitor growth of the cells every 30 minutes by measuring OD at 600nm (OD600) by filling up 750 µL of culture into cuvette. Use LB medium as blank. </li> | ||
| + | <li>Once the cells are ready at OD600 = 0.4-0.6, harvest the cells to prepare for electroporation. </li> | ||
| + | <li>Place cultures on ice for 15 minutes. </li> | ||
| + | <br /> | ||
| + | <p><i>All subsequent steps should be carried out at 4°C.<br />Cells should be kept on ice wherever possible.</i></p> | ||
| + | <br /> | ||
| + | <li>Pour each 250mL culture into pre-chilled 500mL (or 1000mL) Falcon tubes.</li> | ||
| + | <li>Centrifuge at 5000rpm for 10 minutes. </li> | ||
| + | <li>Pour off the supernatant and aspirate any residual broth.</li> | ||
| + | <li>Add 250 mL of pre-chilled 10% (w/v) glycerol to each of the centrifuge bottles and completely suspend the cells by pipetting the solution up and down.</li> | ||
| + | <li>Centrifuge at 5000rpm for 10 minutes. </li> | ||
| + | <li>Pour off the supernatant. </li> | ||
| + | <li>Completely suspend the cells in 250mL glycerol and re-centrifuge at 5000rpm for 10 minutes.</li> | ||
| + | <li>Pour off the supernatant and suspend the cells in the residual glycerol by pipetting up and down.</li> | ||
| + | <li>At this point you can electroporate or freeze the cells away. To freeze, add 100µL of the culture to microcentrifuge tubes on ice. </li> | ||
| + | <li>Once you have used all of the culture, transfer the tubes to dry ice for 10 minutes. </li> | ||
| + | <li>Once the cultures are frozen, transfer them to a -80°C freezer. The cultures should be good for more than 6 months.</li> | ||
| + | </ul> | ||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | <button class="electroporation sub_sub_title">Electroporation</button> | ||
| + | |||
| + | <div class="electroporationinfo"> | ||
| + | <p><i>Ingredients:</i></p> | ||
| + | <ul> | ||
| + | <li><i><a class="SOC" href="#SOCmedium"> SOC medium </a></i></li> | ||
| + | <li><i><a class="LBagar" href="#agar">LB agar plates</a></i></li> | ||
| + | </ul> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 405: | Line 451: | ||
$(".gly").click(function(){ | $(".gly").click(function(){ | ||
$(".glycerolinfo").show(); | $(".glycerolinfo").show(); | ||
| + | }); | ||
| + | }); | ||
| + | |||
| + | $(document).ready(function(){ | ||
| + | $(".SOC").click(function(){ | ||
| + | $(".SOCmediuminfo").show(); | ||
| + | }); | ||
| + | }); | ||
| + | |||
| + | $(document).ready(function(){ | ||
| + | $(".LBagar").click(function(){ | ||
| + | $(".agarinfo").show(); | ||
}); | }); | ||
}); | }); | ||
Revision as of 21:52, 24 September 2016


Medium and Buffers

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare the SOC medium.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.
*Source from 2015 iGEM Exeter
For nucleic acids DNA/RNA separation
- TAE buffer (Tris-acetate-EDTA)
- TBE buffer (Tris-borate-EDTA)
- LAB buffer (Lithium-acetate-borate)

- Prepare the solution by mixing the ingredients stated above.
If not using pre-mixed LB agar powder, prepare the materials as below:
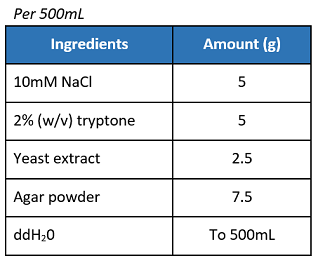
- In a 1L Erlenmeyer flask, swirl and mix the solution.
- Cover the top of the flask with a lid/aluminum foil and label with autoclave tape.
- Autoclave the liquid setting for 20 minutes or according to your autoclave's specifications.
- After removing the solution from the autoclave, allow the agar solution to cool to 55°C in an oven or water bath.
- When pouring the LB agar into plates, keep the bench area sterile by working near a flame or Bunsen burner. Alternatively, prepare the plates in a vacuum hood.
- Add the appropriate amount of desired antibiotic (refer the table below) to the solution and swirl to mix.
- Pour approximately 20mL of LB agar per 10cm polystyrene Petri dish.
- Place the lids on the plates and allow them to cool for until the agar is solidified.
- Label the bottom of plates with antibiotic and date before storing in plastic bags or sealed with Parafilm at 4°C.

Table source from New England Biolabs
Additional note:
- Antibiotic carbenicillin can be substituted for ampicillin in antibiotic selection plates [1].
If not using pre-mixed LB broth powder, prepare the materials as below:

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare SOC medium.
Additional notes
- Some formulations of SOB use 10 mM MgCl2 and 10 mM MgSO4 instead of 20mM MgSO4.
- SOB medium is also available dry pre-mixed from Difco, 0443-17
- Adjust to pH 7.5 prior to use. This requires approximately 25mL of 1M NaOH per liter.
Source from OpenWetWare

- Prepare the solution by mixing the ingredients stated above.
- Filter sterilize using a 0.22 μm filter.
- Store at 4°C or -20°C.
*Source from OpenWetWare: TSS

Transformation
Ingredients:
Before starting the procedure, be sure to:
- Pre-chill centrifuge and cool rotor to 4°C
- Pre-chill 1L of 10% (w/v) glycerol solution on ice
- Pre-chill 500mL/1000mL Falcon tubes on ice
During the procedure:
Work under flame at all times.
- Grow a 5mL overnight culture of cells in LB media.
- In the morning, dilute this culture by at least 1/100 into a 50mL of fresh LB media in a 200mL conical flask.
- Incubate them at 37°C.
- Monitor growth of the cells every 30 minutes by measuring OD at 600nm (OD600) by filling up 750 µL of culture into cuvette. Use LB medium as blank.
- Once the cells are ready at OD600 = 0.4-0.6, harvest the cells to prepare for electroporation.
- Place cultures on ice for 15 minutes.
- Pour each 250mL culture into pre-chilled 500mL (or 1000mL) Falcon tubes.
- Centrifuge at 5000rpm for 10 minutes.
- Pour off the supernatant and aspirate any residual broth.
- Add 250 mL of pre-chilled 10% (w/v) glycerol to each of the centrifuge bottles and completely suspend the cells by pipetting the solution up and down.
- Centrifuge at 5000rpm for 10 minutes.
- Pour off the supernatant.
- Completely suspend the cells in 250mL glycerol and re-centrifuge at 5000rpm for 10 minutes.
- Pour off the supernatant and suspend the cells in the residual glycerol by pipetting up and down.
- At this point you can electroporate or freeze the cells away. To freeze, add 100µL of the culture to microcentrifuge tubes on ice.
- Once you have used all of the culture, transfer the tubes to dry ice for 10 minutes.
- Once the cultures are frozen, transfer them to a -80°C freezer. The cultures should be good for more than 6 months.
All subsequent steps should be carried out at 4°C.
Cells should be kept on ice wherever possible.
Ingredients:

















