| Line 3: | Line 3: | ||
<html> | <html> | ||
| − | . | + | <style> |
| + | .team1 img{ | ||
-webkit-transform: scale(0.5); /* Saf3.1+, Chrome */ | -webkit-transform: scale(0.5); /* Saf3.1+, Chrome */ | ||
-moz-transform: scale(0.5); /* FF3.5+ */ | -moz-transform: scale(0.5); /* FF3.5+ */ | ||
| Line 11: | Line 12: | ||
} | } | ||
| − | + | ||
.divindiv{ | .divindiv{ | ||
margin:auto; | margin:auto; | ||
Revision as of 20:14, 23 September 2016


Medium and Buffers

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare the SOC medium.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.
*Source from 2015 iGEM Exeter [link = 2015.igem2015 .org/wiki/images/a/ab/Exeter_Glycerol_recipe.pdf]
For nucleic acid DNA/RNA separation
- TAE buffer (Tris-acetate-EDTA)
- TBE buffer (Tris-borate-EDTA)
- LB buffer (Lithium borate)

- Prepare the solution by mixing the ingredients stated above.
If not using pre-mixed LB agar powder, prepare the materials as below:
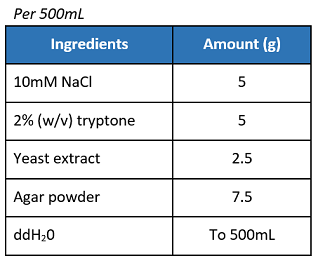
- In a 1L Erlenmeyer flask, swirl and mix the solution.
- Cover the top of the flask with a lid/aluminum foil and label with autoclave tape.
- Autoclave the liquid setting for 20 minutes or according to your autoclave's specifications.
- After removing the solution from the autoclave, allow the agar solution to cool to 55°C in an oven or water bath.
- When pouring the LB agar into plates, keep the bench area sterile by working near a flame or Bunsen burner. Alternatively, prepare the plates in a vacuum hood.
- Add the appropriate amount of desired antibiotic (refer the table below) to the solution and swirl to mix.
- Pour approximately 20mL of LB agar per 10cm polystyrene Petri dish.
- Place the lids on the plates and allow them to cool for until the agar is solidified.
- Label the bottom of plates with antibiotic and date before storing in plastic bags or sealed with Parafilm at 4°C.
















