| Line 602: | Line 602: | ||
While, inhibition circle was observed on the plate with farnesol (B).]] | While, inhibition circle was observed on the plate with farnesol (B).]] | ||
<br /> | <br /> | ||
| − | This result indicates that farnesol affect E.coli. So, we ( | + | This result indicates that farnesol affect E.coli. So, we (麻生hypothesis) that E.coli needs to have resistance to farnesol. |
<br /> | <br /> | ||
[[File:E.colimarA result .jpg|200px|thumb|center|Fig.7:Colony formation of E. coli JM109 engineered with marA on farnesol overlaid plates. | [[File:E.colimarA result .jpg|200px|thumb|center|Fig.7:Colony formation of E. coli JM109 engineered with marA on farnesol overlaid plates. | ||
Revision as of 12:11, 16 October 2016
Result and Discussion
Confirmation antibacterial activity of farnesol.
First, we examined our working hypothesis to “Flavorator” that farnesol can show either the antibacterial or bacteriostatic activity in a box like “KOZOKO”. The results clearly showed that farnesol had antibacterial properties. In the literatures, farnesol have antibacterial volatiles. Farnesol is produced after complicated pathways, so for their syntheses, various enzymes are required. In E. coli, farnesol may be synthesized. In this context, we designed our system for establishing the concept of “Flavorator” to build up a brand-new biosynthetic pathways, in which farnesol is produced in the E. coli. In doing so, we transfered the three types of genes listed below to create the hyper-producer E. coli of farnesol. We examined our working hypothesis to “Flavorator” that farnesol can show either the antibacterial or bacteriostatic activity in a box like Kozoko.
Confirmation of anti-mold, anti-maggots and antibocatrial activities of farnesol.
E. coli can easily synthesize antimicrobial volatiles farnesol and geraniol. We examined the effects of three antimicrobial volatiles against bacteria which rot food.

A: geraniol(100 μl of geraniol solution was dropped on the cotton)B:farnesol(100 μl) C: ethanol (100 μl ) A:Chopstick dipped in suspension of mold was touched in the center of the bread. The volume of box is 846cm³. B:The same treatment was done. C:The same treatment was done. These boxes were kept for 5 days at room temperature.
Farnesol had the highest antifungal activity against mold.
We found that farnesol has high antifungal activity against the mold of bread. Therefore, we investigated whether farnesol exerts similar antifungal effects on other food.

A: farnesol(1ml of farnesol solution was dropped on the cotton), B:ddH2O(1ml). A:Chopstick dipped in suspension of mold was touched in the center of the bread. The volume of box is 846cm³. B: The same treatment was done. These boxes were kept for 8 days at room temperature.
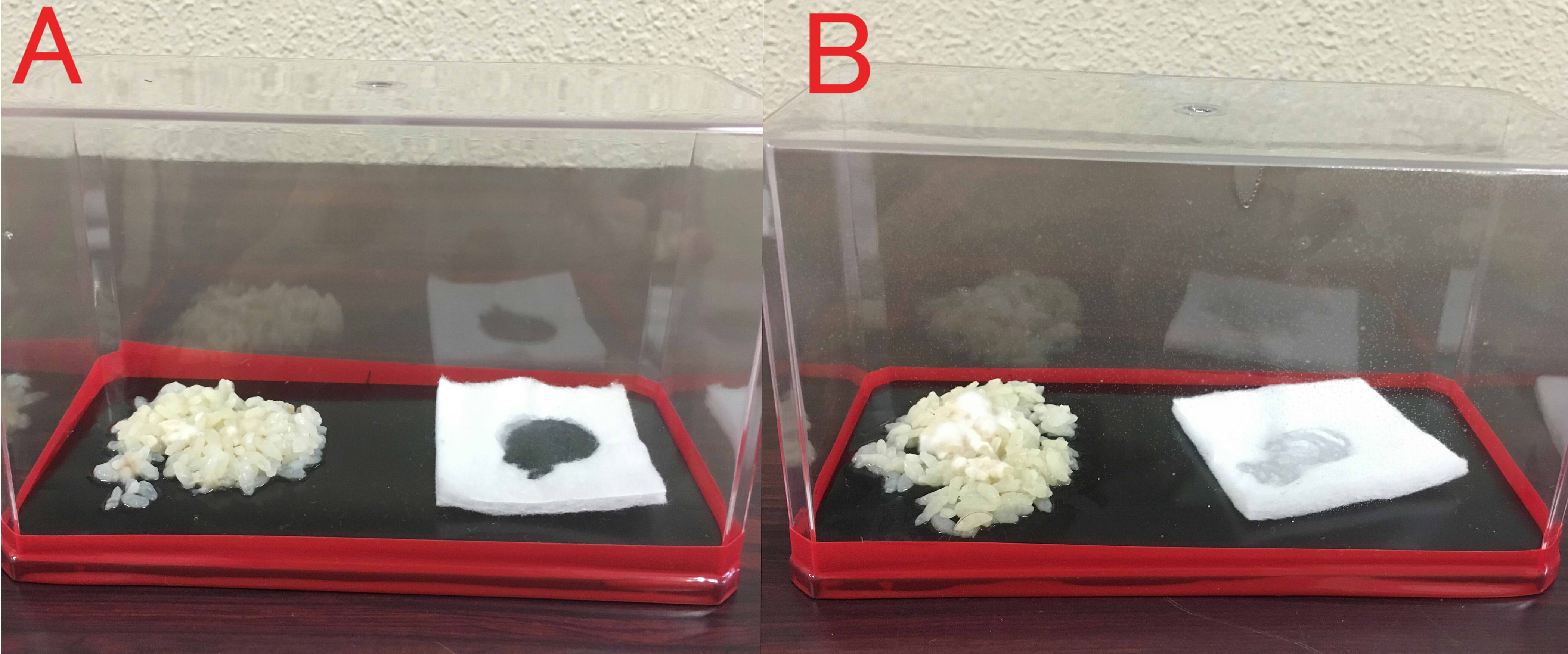
A: farnesol(1ml of farnesol solution was dropped on the cotton), B:ddH2O(1ml). A:Chopstick dipped in suspension of mold was touched in the center of the rice. The volume of box is 846cm³. B: The same treatment was done. These boxes were kept for 8 days at room temperature.

A: farnesol(1ml of farnesol solution was dropped on the cotton), B:ddH2O(1ml). A:Chopstick dipped in suspension of rotten meat was touched in the center of the chicken. The volume of box is 846cm³. B: The same treatment was done. These boxes were kept for 8 days at room temperature.
We found that farnesol has a preservative effect on various foods.
We examined whether farnesol also has an effect against food poisoning bacteria. We used Staphylococcus aureus as food poisoning bacteria. We examined whether farnesol inhibits their growth.
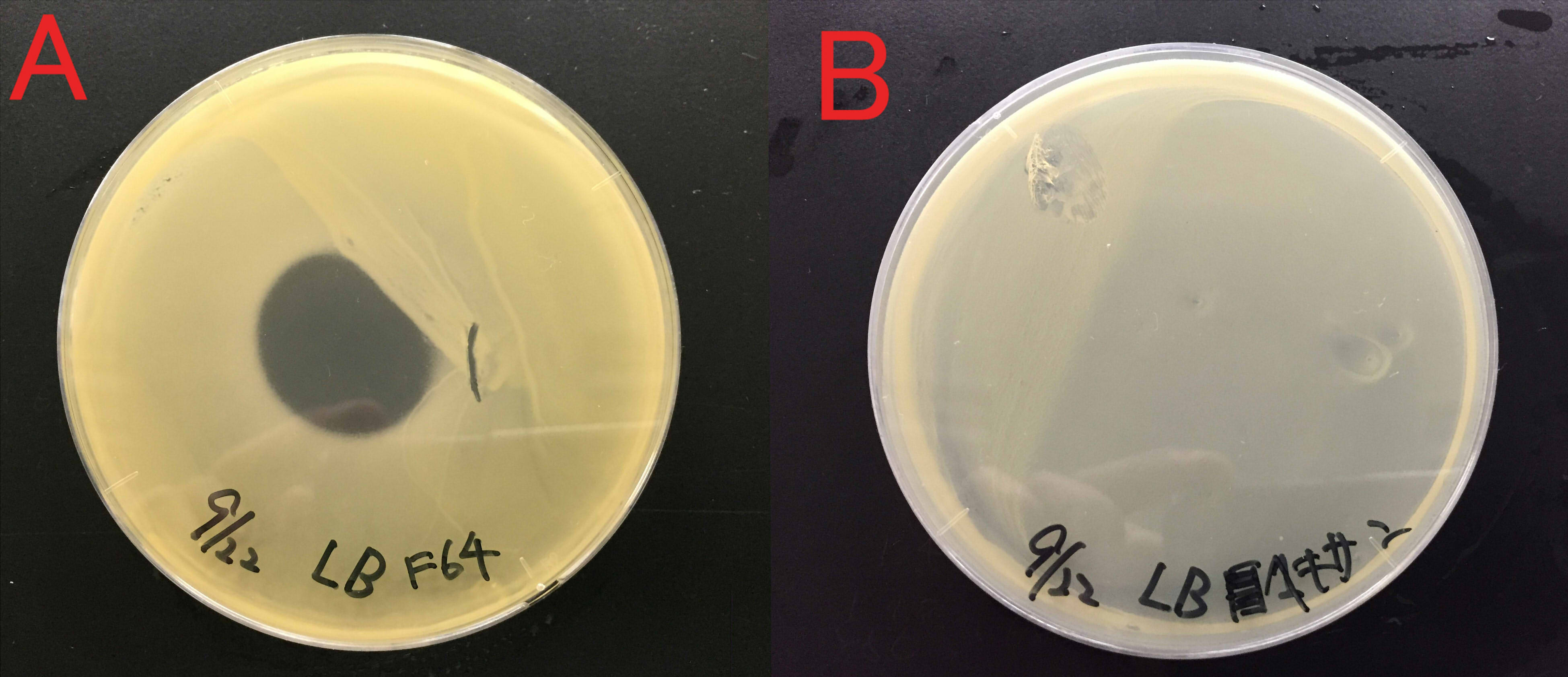
A:Farnesol with ddH2O(300μl) B:ddH2O(300μl). Farnesol was dropped on the paper which was put on center of the plate cover without direct contact with the bacteria. These plates were incubated for 21 hours at 37 ℃. Growth inhibition circle was not observed on the plate without farnesol (B). While, inhibition circle was observed on the plate with farnesol (A). These results indicate that it is difficult for Staphylococcus aureus to grow in the presence of farnesol.
We considered that farnesol has high effect against inhibitory the growth food poisoning bacteria because diluted farnesol affect staphylococcus aureus.
We thought about a possibility that farnesol may affect E.coli, which produces farnesol, because E. coli is also a bacterium. Therefore, we examined whether farnesol affects E.coli.
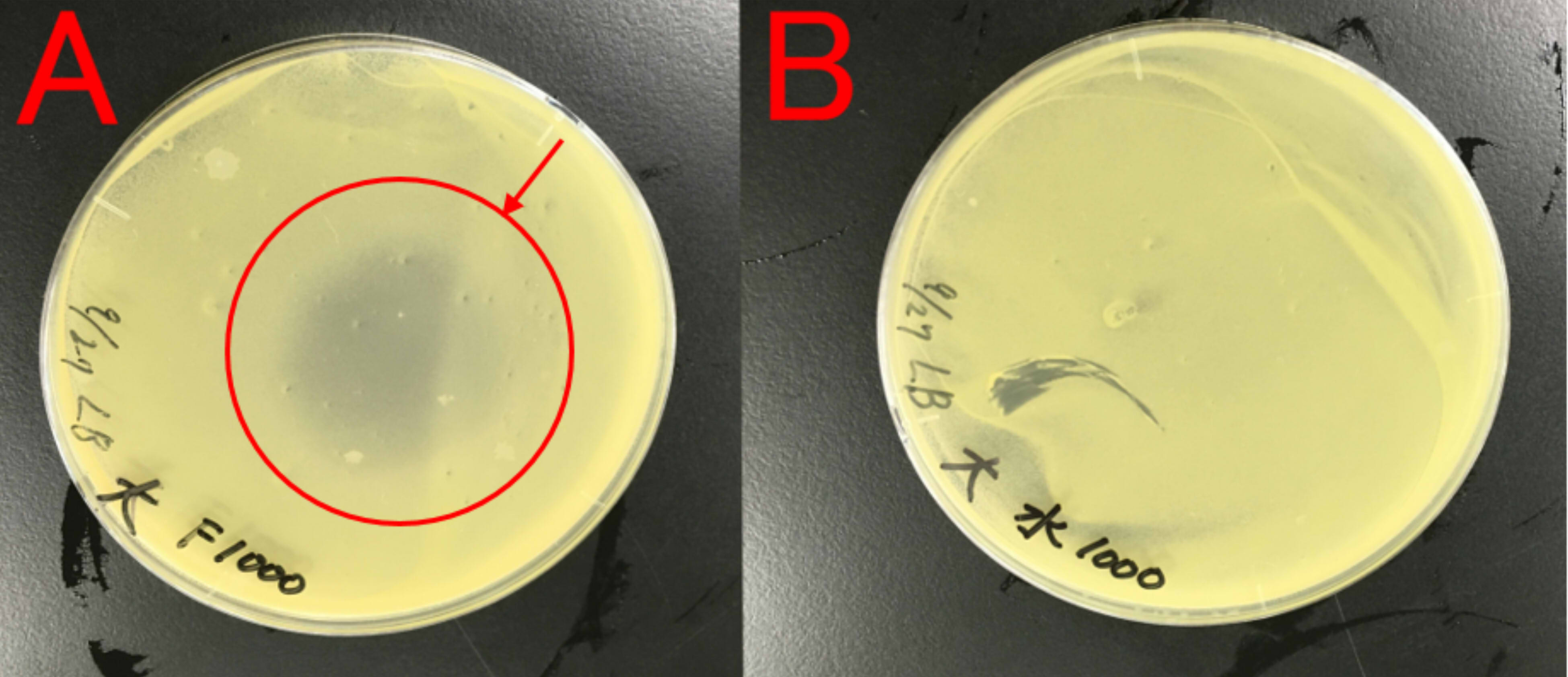
A:ddH2O (1ml) B:farnesol (1ml) Farnesol was dropped on the paper which was put on center of the plate cover without direct contact with the bacteria. These plates were incubated for 21 hours at 37 ℃. Growth inhibition circle was not observed on the plate (A). While, inhibition circle was observed on the plate with farnesol (B).
This result indicates that farnesol affect E.coli. So, we (麻生hypothesis) that E.coli needs to have resistance to farnesol.
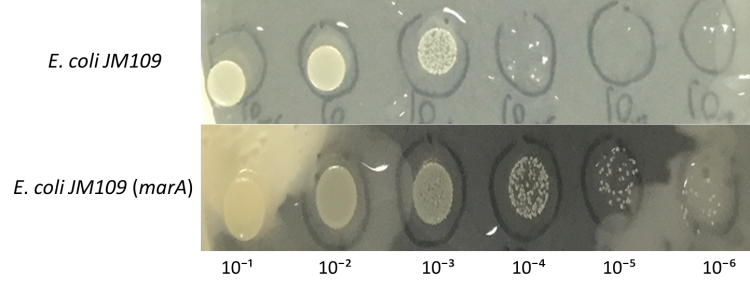
E. coli JM109 and E. coli JM109 (marA) were spotted on LBGMg agar plates in serial ten-fold dilutions (10⁻¹~〖10〗^(-6)), overlaid with 30 % (v/v) farnesol hexane solution (farnesol solution) and incubated at 30°C for 24 h. This figure shows that E. coli JM109 (marA) cells that overexpress the marA product better than the control E. coli JM109 wild type cells survived plates overloyed by 30 % farnesol solution.