| Line 142: | Line 142: | ||
<table cellpadding="0" cellspacing="0"> | <table cellpadding="0" cellspacing="0"> | ||
| − | + | [[File:T--FAFU-CHINA--mo2.png | 825px | thumb | center ]]<tr><td width="0" height="0"></td></tr><tr><td></td><td></td></tr> | |
<span style="font-family:'Times New Roman';font-size:12.0000pt"> </span><p></p> | <span style="font-family:'Times New Roman';font-size:12.0000pt"> </span><p></p> | ||
<p style="text-indent:24pt"><span style="font-family:'Times New Roman';font-size:12.0000pt"> </span></p> | <p style="text-indent:24pt"><span style="font-family:'Times New Roman';font-size:12.0000pt"> </span></p> | ||
Revision as of 08:19, 18 October 2016
Protein Structure- Supported by SCU-CHINA
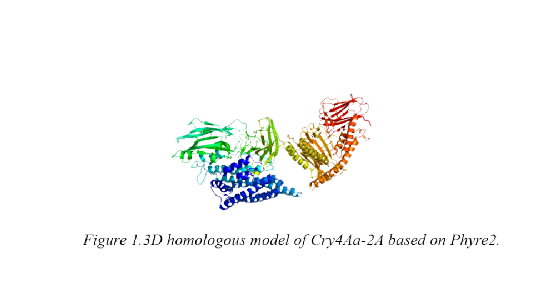
In 2016 iGEM competition, FAFU-CHINA aims to use the combination of Cry and Cyt toxins to control mosquito larvae in order to decrease the disease transition like Malaria and Zika from mosquito. 2A peptides is an effective tool to co-express two different proteins with one single vector. However, when 2A peptides is cleaved during the translation, residues with a length of about 20 amino acids are remained at the end of the upstream protein and a proline remained at the beginning of the downstream protein. Because of this, we are not sure about whether the residues would affect the structure and function of the proteins that we are going to express in Chlamydomonas reinhardtii, especially the Cry toxins. Therefore, SCU-CHINA team helped Tong Tong used Phyre2 to predict the secondary and tertiary structure of Cry4Aa, Cry10Aa and Cry11Aa with 2A residues. Unfortunately, the structure of Cry10Aa and Cry11Aa have not been elucidated by using X-ray crystallographic methods. Hence there is no good template in PDB database can be used and a large number of errors are reported from the results of homologous model construction for Cry10Aa-2A and Cry11Aa-2A. We were unable to estimate the effects of 2A residues to these two Cry toxins. However, the structure of Cry4Aa has been reported since 2006. We submitted the amino acid sequence of Cry4Aa-2A to Phyre2 website, using the normal mode and obtained a series of result of the homologous model. We got a reliable homologous model of Cry4Aa-2A (Fig. 1).
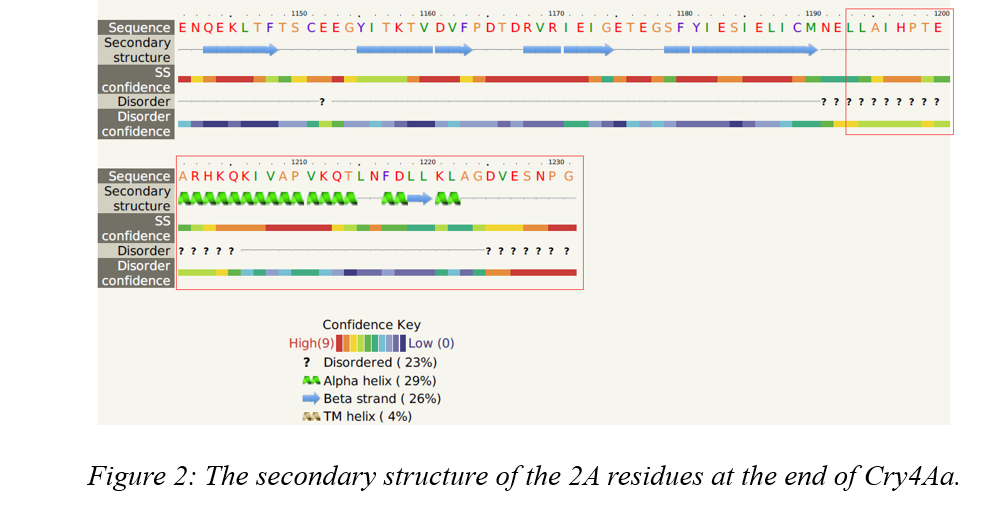
We also obtained secondary structure analysis from the result of Phyre2. Since the 2A residues structure which has a length of 39 aa is our interest, so we carefully looked into that and found about half of residues are disordered and another half are mainly α-helix and a short β-strand (Fig. 2). Thus, we have reason to believe that the 2A residues does little impact to our Cry4Aa toxin, and it's feasible to use 2A peptides as a link between Cry and Cyt toxin for co-expression in a single vector.