| Line 178: | Line 178: | ||
<p><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> </span></p> | <p><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> </span></p> | ||
<p><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> </span><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt">Therefore, we decided to construct the expression system to co-express </span><i><span style="font-family:'Times New Roman';color:rgb(34, 34, 34);font-size:12pt">Cry</span></i><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> and </span><i><span style="font-family:'Times New Roman';color:rgb(34, 34, 34);font-size:12pt">Cyt</span></i><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> genes in single vector based on 2A peptide system.</span><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"></span></p> | <p><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> </span><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt">Therefore, we decided to construct the expression system to co-express </span><i><span style="font-family:'Times New Roman';color:rgb(34, 34, 34);font-size:12pt">Cry</span></i><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> and </span><i><span style="font-family:'Times New Roman';color:rgb(34, 34, 34);font-size:12pt">Cyt</span></i><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> genes in single vector based on 2A peptide system.</span><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"></span></p> | ||
| − | == <span style="font-family:'Times New Roman';color:rgb(51, 51, 51)">7.Toxin Groups</span><span style="font-family:'Times New Roman';color:rgb(51, 51, 51);font-size:14pt"></span> == | + | == <p><b><span style="font-family:'Times New Roman';color:rgb(51, 51, 51)">7.Toxin Groups</span></b><b><span style="font-family:'Times New Roman';color:rgb(51, 51, 51);font-size:14pt"></span></b></p> == |
<p><b><span style="font-family:'Times New Roman';color:rgb(34, 34, 34);font-size:12pt"> </span></b><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt">As mentioned in the </span><i><span style="font-family:'Times New Roman';font-size:12pt">Action mode of Cry and Cyt toxins</span></i><span style="font-family:'Times New Roman';font-size:12.0000pt">, </span><i><span style="font-family:'Times New Roman';font-size:12pt">Cyt </span></i><span style="font-family:'Times New Roman';font-size:12.0000pt">can increase the toxicity of </span><i><span style="font-family:'Times New Roman';font-size:12pt">Cyt.</span></i><span style="font-family:'Times New Roman';font-size:12.0000pt"> Therefore, we designed various toxin groups based on the 2A-peptide system as following </span><i><span style="font-family:'Times New Roman';font-size:12pt">figure8</span></i><span style="font-family:'Times New Roman';font-size:12.0000pt">.</span><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"></span></p> | <p><b><span style="font-family:'Times New Roman';color:rgb(34, 34, 34);font-size:12pt"> </span></b><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt">As mentioned in the </span><i><span style="font-family:'Times New Roman';font-size:12pt">Action mode of Cry and Cyt toxins</span></i><span style="font-family:'Times New Roman';font-size:12.0000pt">, </span><i><span style="font-family:'Times New Roman';font-size:12pt">Cyt </span></i><span style="font-family:'Times New Roman';font-size:12.0000pt">can increase the toxicity of </span><i><span style="font-family:'Times New Roman';font-size:12pt">Cyt.</span></i><span style="font-family:'Times New Roman';font-size:12.0000pt"> Therefore, we designed various toxin groups based on the 2A-peptide system as following </span><i><span style="font-family:'Times New Roman';font-size:12pt">figure8</span></i><span style="font-family:'Times New Roman';font-size:12.0000pt">.</span><span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"></span></p> | ||
<p>[[File:T--FAFU-CHINA--e5n.png | 825px | thumb | center ]]<span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> </span></p> | <p>[[File:T--FAFU-CHINA--e5n.png | 825px | thumb | center ]]<span style="font-family:'Times New Roman';color:rgb(34,34,34);font-size:12.0000pt"> </span></p> | ||
Revision as of 22:21, 19 October 2016
Contents
- 1 1.Introduction
- 2 2.The Damage of Mosquitoes
- 3 3.The Life Cycle of Mosquitoes
- 4 4.Action mode of Cry and Cyt toxins
- 5 5.The shortcomings of Bacillus thuringiensis (Bt.)
- 6 6.2A-Expression system
- 7 <p><b>7.Toxin Groups</b><b></b></p>
- 8 8.The Protein Express Platform in Chlamydomonas reintmrdtii
- 9 9.The Culture and Transformation of Chlamydomonas reintmrdtii
- 10 10.Bioassay of Toxins Express Devices
- 11 11.Co-Immunoprecipitation Experiment (BNU-China Collaboration)
- 12 12.Western Blot of tCas9-CIBN Expression (NEU-China
Cry for Mosquitoes: A New Way to Control Mosquitoes by Synthesis Biology
Abstract: In 2016, we focused on to control mosquitoes. We cloned various Cry and Cyt toxin genes from LLP29, Bacillus thuringiensis strain. We also constructed Cry and Cyt toxin parts based on option optimization of Chlamydomonas reinhardtii. Meanwhile, we built toxin groups express device based on 2A peptide system. And we tested their toxicity to the larvae of various genus of mosquitoes under the safe lab environment. Considering the potential problems in the natural environment, BNU-China and ZJU-China helped us to build the growth and toxicity model to estimate the natural conditions. To introduce the practical knowledge about how to prevent and control mosquitoes, we designed a handbook covering this topic and sent it to Zambia to help local people who are trouble in mosquitoes. We are proud of our project!
1.Introduction
Mosquitoes are one of the most harmful insects in the world because they bite animals and human and spread virus. How to control mosquitoes in an efficient, green and cheap way has been a worldwide problem. This year, FAFU-CHINA iGEM team decide to start with the food source to control the amount of mosquitoes.
According to preliminary researches about physiological characters of mosquitoes larvae. They feed on algae, plankton, fungi and bacteria and other microorganisms. Bacillus thuringiensis (Bt.) is a Gram-positive bacterium which commonly used as biological pesticide. Based on the previous research in Fujian Agriculture and Forestry University, LLP29 is a bacterial Bt. which isolated from the natural environment in Mountain Wuyi. LLP29 has been proved the specific and efficient toxicity to Mosquitoes larvae by bioassay. But the Bt. owns fatal weakness that they cannot live for a long time in the natural environment, especially when they are under sunshine exposure. The heterologous expression of toxins in suitable organism will be a good choice to solve this problem. Saccharomyces cerevisiae is a general choice for heterologous expression in eukaryote. But to decrease the cost of this method, we decide to make use of photosynthetic organism which can live in water. Chlamydomonas reinhardtii is a unicellular alga which belongs to the Chlorophyte Phylum. It can be the food source for mosquitoes larvae. According to the result of identification and clone from LLP29, Cry and Cyt proteins are the main resource of toxicity. According to the previous research, Cry proteins can improve the toxicity of Cyt proteins by combination. Therefore, we chose 2A-peptide expression system to co-express Cry and Cyt proteins.
2.The Damage of Mosquitoes
Mosquitos are the one of the most harmful in the world. They can spread various mosquito-borne by biting animals and human. Nowadays, epidemic diseases like Zika, dengue and malaria that are transmitted by mosquitoes have infected human seriously and tended to distribute around the world.
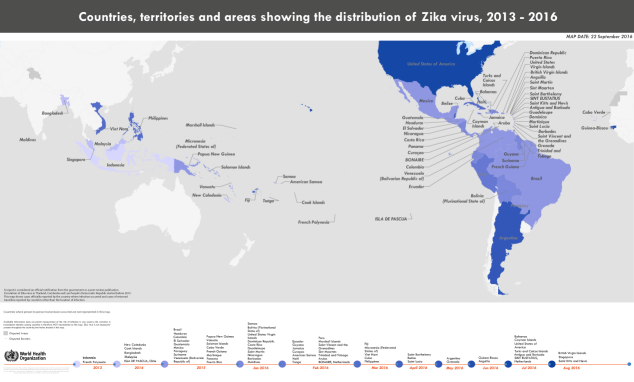
Take Zika for instance, it is spread mostly by the bite of an infected Aedes species mosquito (Ae. Aegyptus and Ae. Albopictus). These mosquitoes bite human and animals during the day and night. [1] Mother-baby transmission, sex and blood can also transmit the Zika virus. According to the latest Zika situation report of WHO, since 2015, mosquito-borne Zika virus infections have been evidenced in 73 countries or territories including 56 areas have ever occurred outbreak as Figure0. And all the continents have reported infection cases. There is no need to emphasize the damage of Zika virus because the news about damage has been known around the world by media reporters.
Recently, America and Brazil have agreed to strengthen cooperation on research of Zika vaccine. But actually, it takes a long time for the drug development. Therefore, the best way to reduce the spread of Zika is to kill the vector mosquitoes. In addition, CDC (Center for Diseases Control and Prevention) suggests that we can
(1) use EPA (Environmental Protection Agency (EPA) registered insect repellent
(2) wear long-sleeved shirts and long pants
(3) stay in places with air conditioning or window and door screens
(4) remove standing water around your home,
5) as for pregnant women, they should avoid travelling to epidemic areas.
Figure0: Countries, territories and areas showing the distribution of Zika virus,2013-2016
Dengue is the most epidemic virus in the tropics and subtropics area. Dengue is now epidemic around over 100 countries including Africa, America, Mediterranean, Southeast Asia and Western Pacific areas. One research estimated that about 3.9 billion people may be infected with dengue fever yearly and 96 million people included may have serious or mild clinical symptoms. Actually, dengue can infect not only babies, children but also adults, but cannot lead to death commonly. Undoubtedly, the most effective protective measures are those that avoid mosquito bites. The best way to reduce mosquitoes is to eliminate the places where the mosquito lays her eggs, like artificial containers that hold water in and around the home. Outdoors, clean water containers like pet and animal watering containers, flower planter dishes or cover water storage barrels. Look for standing water indoors such as in vases with fresh flowers and clean at least once a week. [2]
Malaria is another mosquito-borne infectious disease. It is caused by four Plasmodium species: Plasmodium falciparum – malignant tertian, Plasmodium vivax – benign tertian, Plasmodium ovale – benign tertian, Plasmodium malariae – quartan malaria. All are transmitted by female Anopheles mosquitoes. Malaria occurs mostly in poor tropical and subtropical areas of the world. Plasmodium falciparum is the most common Plasmodium in the African continent, which leads to majority death cases all over the world. And Plasmodium vivax is more widespread that Plasmodium falciparum, epidemic both Africa and other areas. According to the report from WHO, it is reported that about 3.2 billion people (nearly half of the global population) are at the risk of malaria. Now, malaria is spreaded in about 95 countries or territories. Due to lacking vaccine, the most effective way to eliminate malaria is to kill the specific mosquito female Anopheles.
Although we have tried our best to prevent human from these epidemic diseases, the global risk assessment has not changed. Hence, what we do now is to discover a suitable way to kill the special mosquitoes.
3.The Life Cycle of Mosquitoes
Mosquito is a complete metamorphosis insect. The life cycle from egg to adult is about 7 to 10 days. The mosquito goes through four separate and distinct stages of its life cycle: Egg, Larva, Pupa and Adult. Each of these stages can be easily recognized by its special morphological and physiological characteristics as Figure1-4.
4.Action mode of Cry and Cyt toxins
Sufficient evidence has proven that the Cry and Cyt protein family from Bt., produced as parasporal crystal components, can be greatly effective against insects including Lepidoptera, Coleoptera, Diptera and nematodes. By forming pores on the cell membrane or inserting into membrane, these proteins can lyse insects' midgut epithelial cells, and then result in the death. However, proteins from both of the Cry and Cyt families are harmless to human,other vertebrates and plants. In addition, they are completely biodegradable which lessen the potential hazard to our environment. Therefore, people are widely using Bt. to control pests in order to prevent the loss of agricultural production and spread of human disease.
Cry4Aa, Cry10Aa, Cry11Aa, Cry4b, Cyt1Aa and Cyt2 toxins from Bt ssp. israelensis (Bti.) have great toxic effect on mosquito larvae. Different types of Cry proteins' toxicity to different species of mosquitoes can vary a lot. For example, Cry4b shows no toxicity to Culex while Cry4Aa does. However, mutagenesis of certain region of Cry protein can make it show toxicity to the species it never has before.
The Cry protein is consisted of three functional domains. Domain I is a seven α-helices bundle. It can insert itself into a membrane by using its hydrophobic helices α4 and α5 to insert into the phospholipid bilayer. The pore formation occurs on its α3 helix. Domain II and domain III are two β-sheets which are involved in the receptor interactions. Domain II contains extremely variable loops, which are the binding site of the receptor. Domain III has the function of stabilizing the toxin.
Cyt proteins have a single α-β domain which do not bind to receptors but can directly insert into the cell membrane and then form a pore causing cell death. Although Cry and Cyt proteins are two big families of δ-endotoxins, they are far related. Cyt1 and Cyt2 are two types of Cyt proteins found in Bti..
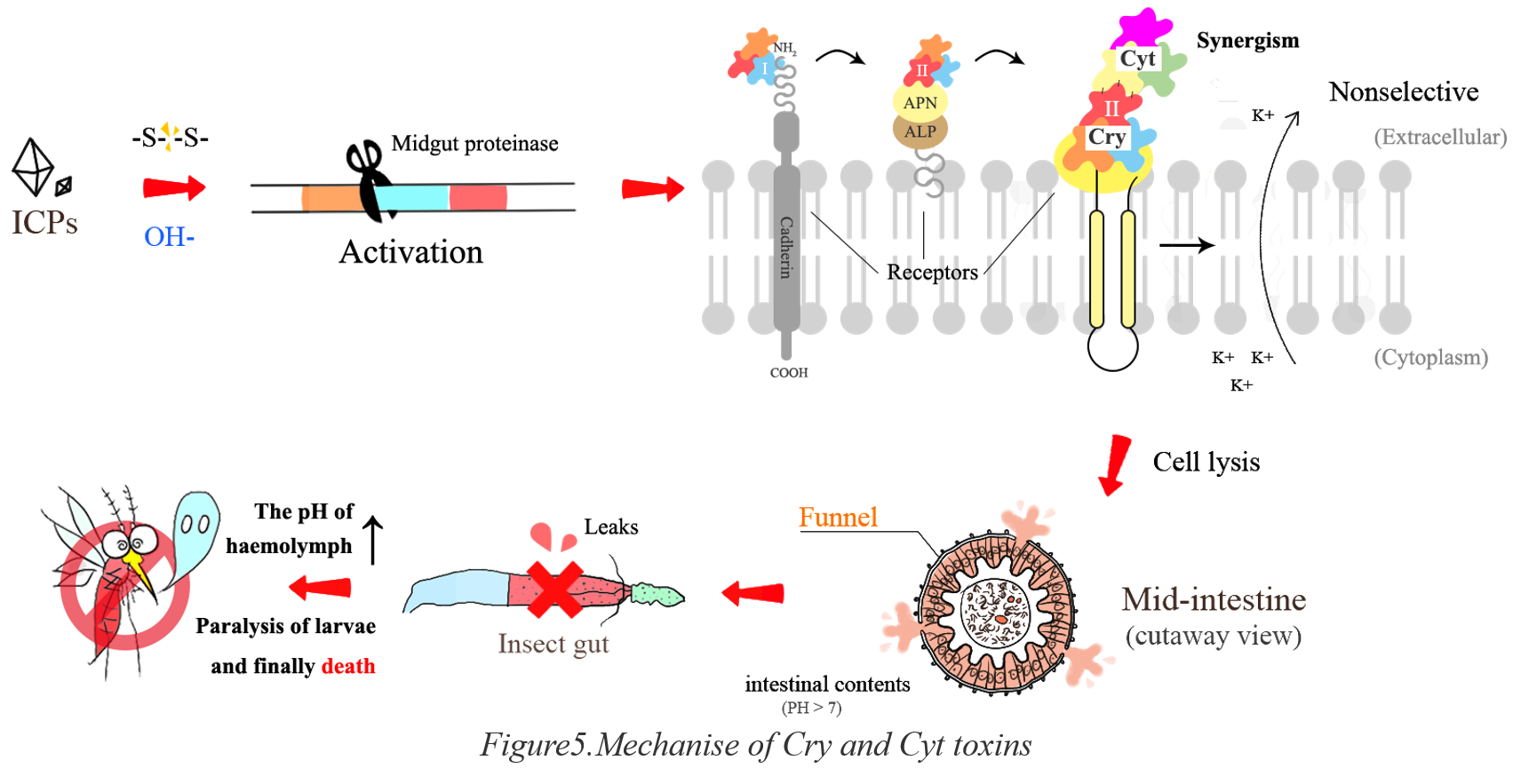
Generally, Cry proteins are believed to exert toxicity by interacting with the proteins on the brush border membrane and then insert into the membrane which takes multiple steps. At the beginning in mosquitoes' gut, the crystalline inclusions are cleaved at the disulfide bond to release the Cry pre-toxin. Then the soluble proteins are activated by being cleaved again by intestinal protease. When toxins reach to the brush border membrane microvilli, they bind to the proteins, or known as receptors on the membrane. The binding process takes two step. Firstly, the monomeric Cry toxin binds to cadherin, resulting in the formation of pre-pore oligomer as Figure5.
Then the oligomer binds to a GPI-anchored APN or ALP. Secondly, the previous binding induces the oligomer insertion into the lipid rafts membrane. A formation of ion permeable pore is followed by the insertion which allows small molecules to pass through the membrane. The membrane potential inevitably changes greatly, causing the swelling of cell and finally breaking down. When the cell lysis reaches to a certain degree, the midgut necrosis and epithelial denaturation follow. Then, the alkaline hypertonic inclusions in midgut enters into hemocoel and the pH of haemolymph rises causing paralysis of larvae and finally death.
However, there are evidences shown that there is another model of pore formation caused by Cry toxin action in Lepidoptera. The interaction between Cry toxin and cadherin can trigger the programed death of by lysis.
5.The shortcomings of Bacillus thuringiensis (Bt.)
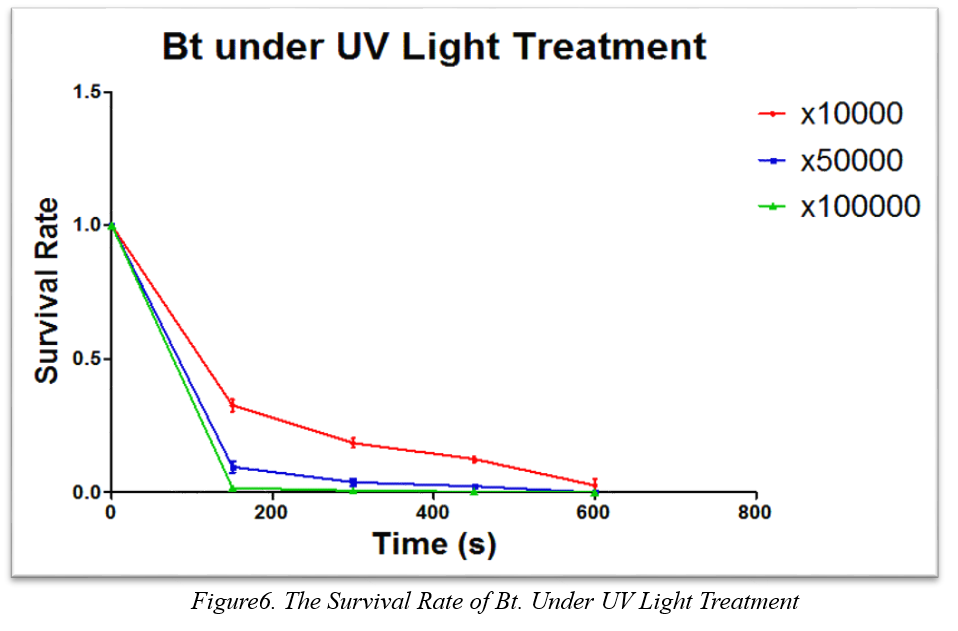
<p style="text-indent:36pt">There is no doubt that Bt. stains own many advantages as biological pesticide, such as, efficiency and highly species-specific toxicity. Howerver, B. thuringiensis also does have disadvantages. Many strains are sensitive to the sunlight and their toxic proteins will degrade within a week. </p> <p style="text-indent:36pt">To test the resistance of LLL29 to the sunshine, especially about UV, we measure the curve of survival as Figure6 under the different time length under different illumination time by using UV as Figure6.</p>
6.2A-Expression system
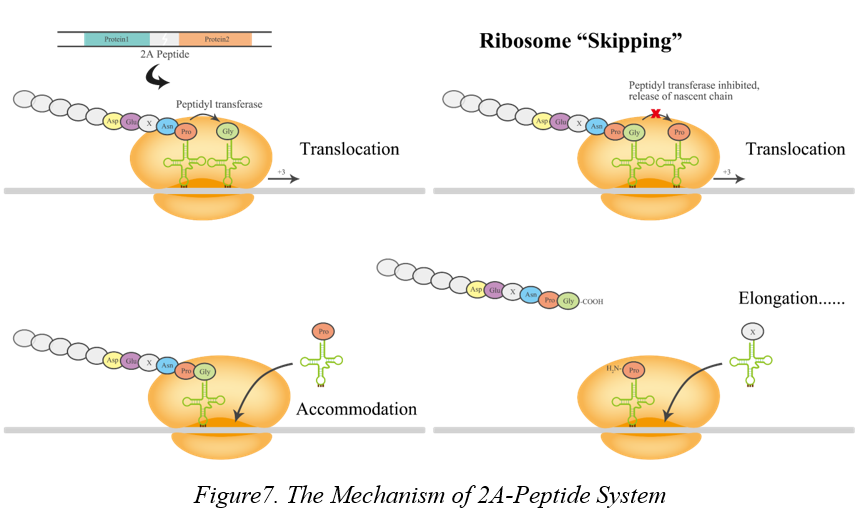
<p> The interaction between Cry and Cyt toxins can enhance the toxicity to mosquitoes. Therefore, it is essential for us to develop a system which co-expressed Cry and Cyt genes together to kill the larvae of mosquitoes. For this system, the expression of multiple proteins is the most critical stage in synthesis biology. From previous studies, we found that an internal ribosomal entry site (IRES) has been widely used to construct bicistronic or multicistronic vectors. But, due to the unbalanced expression of upstream and downstream proteins and the limit of their sequence lengths, we proposed a new strategy based on 2A peptide expression system. This system is based on a self-cleaving 2A peptide and much more efficient to cleave the upstream of 2A peptide. Comparing to the widely used method, multiple plasmids for transformation, Viral 2A peptide system not only works in Escherichia coli and Saccharomyces cerevisiae, but also performs much more efficiently than that of plasmids, especially in C. reinhardtii.</p> <p> 2A peptides system was firstly discovered in Foot-and-mouth disease virus (FMDV). 2A peptide is a linker which used to separate functional proteins between upstream and downstream, and the length is about 18-22 amino acids long. 'Ribosomal skip' occurs during translation that results in production of independent peptides or proteins from one polycistronic. After cleavage, the 2A peptide remains fused to the C-terminus of the upstream protein when the proline is linked to the N-terminus of the downstream as Figure7. According to the Thomas's research, 2A peptide system has proved the high-efficiency of co-expression and cleavage in Chlamydomonas reinhardtii. </p>
<p> </p> <p> Therefore, we decided to construct the expression system to co-express Cry and Cyt genes in single vector based on 2A peptide system.</p>
<p><b>7.Toxin Groups</b><b></b></p>
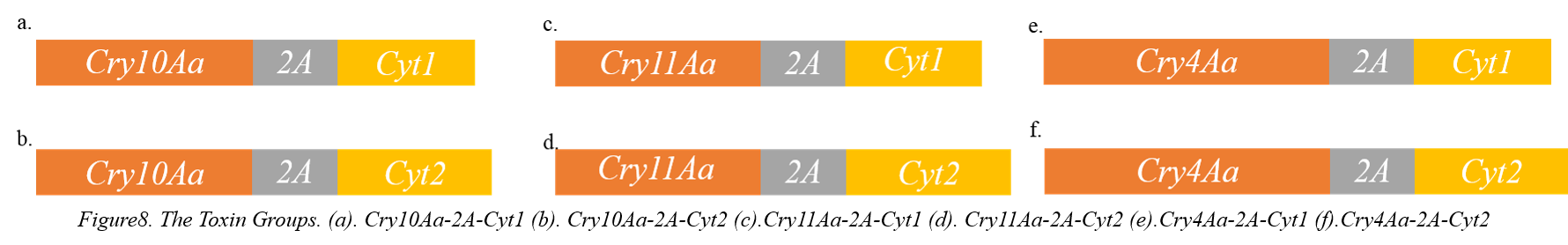
<p><b> </b>As mentioned in the Action mode of Cry and Cyt toxins, Cyt can increase the toxicity of Cyt. Therefore, we designed various toxin groups based on the 2A-peptide system as following figure8.</p>
<p> </p><p style="text-indent:36pt">Considering the bias of codon in Chlamydomonas reintmrdtii, the synthesis biology company helped us to synthesis the Cry10Aa-2A-Cyt1, Cry10Aa-2A-Cyt2, Cry11Aa-2A-Cyt1, Cry11Aa-2A-Cyt2, Cry4Aa-2A-Cyt1, Cry4Aa-2A-Cyt2 toxin gene groups.</p>
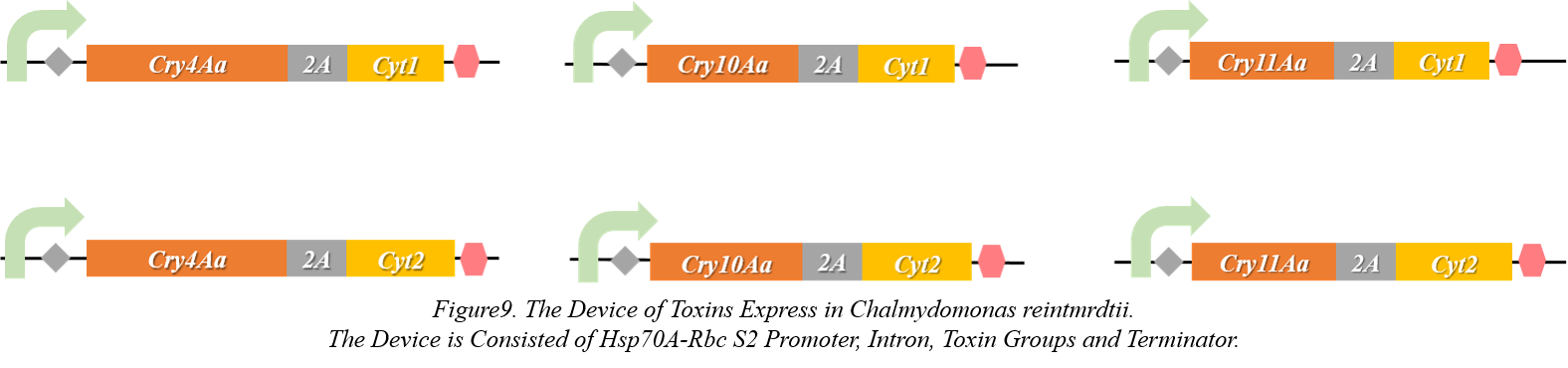
8.The Protein Express Platform in Chlamydomonas reintmrdtii
<p><b> </b>Generally, E. Coli and Saccharomyces cerevisiae are good platforms to express proteins from eukaryon or prokaryote. But considering the cost of our product in the natural world. Algae will be a better choice for us because they can make use of sunshine to produce proteins by photosynthesis. Chlamydomonas reintmrdtii is a typical genus of green alga. The integrating data of genome, high efficiency of protein express and mature systems of culture and transform. Therefore, we constructed six toxin express devices as Figure9.
</p>
9.The Culture and Transformation of Chlamydomonas reintmrdtii
<p style="text-indent:36pt">The most transformation is based on electro transformation technology. But due to the unknown reasons, we still could not transform Hsp70A-Rbc S2 Promoter-Intron-Cry10Aa-2A-Cyt-Terminator device into Chlamydomonas reintmrdtii. With the help from SZU-China, they helped us to transform it by glass-bead technology. You also can find the culture and transformation protocols in the Protocol page. </p>
10.Bioassay of Toxins Express Devices
<p style="text-indent:36pt">After building toxins express devices and transforming them into Chlamydomonas reintmrdtii, we decided to test the toxicity to the larvae of mosquitoes. You can find the Bioassay process and result in the Proof of Concept. You also can visit this link: https://2016.igem.org/Team:FAFU-CHINA/Proof</p>
11.Co-Immunoprecipitation Experiment (BNU-China Collaboration)
<p style="text-indent:21.0000pt">BNU-CHINA wanted to detect paclitaxel by using microtubule which expressed in E. coli. As we all know, microtubule which assembled by α-subunit and β-subunit is the cytoskeleton in eukaryotes, but E. coli is prokaryote. So it is essential to verify whether microtubule assembled successfully or not in E. coli. If microtubule assembled successfully, that’s meaningα-subunit and β-subunit have biological activities and have interaction in E. coli. So, our team planned to use Co-Immunoprecipitation(Co-IP) to confirm their interaction.</p> <p style="text-indent:21.0000pt">Because PET30a has the His protein tag, we planned to add HA protein tag and Flag protein tag behind α-subunit and β-subunit respectively. The target gene was amplified by PCR and cloned to T-vector and sequenced. Then link the confirmed gene to PET30a (enzyme site: XhoI and HindIII). Transform the recombination vector into expression strains BL21 when the concentration of bacterium is appropriate (OD600 is 0.6-0.8). Induce the strains by 1mmol IPTG. By using ultrasonic waves to break the cell of strain and centrifuging, we obtain the deposit. To conduct the Co-Immunoprecipitation(Co-IP), we deal with the Inclusion body to get the protein.</p>
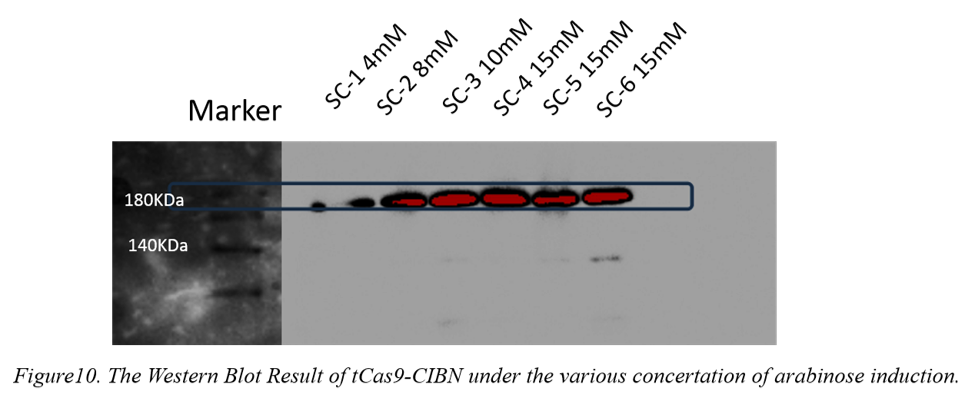
12.Western Blot of tCas9-CIBN Expression (NEU-China
<p style="text-indent:36pt">When we receive help from NEU-China team to help us to get the confocal photo of eGFP express in Chlamydomonas reintmrdtii, we also helped them to repeat their induction experiment. We culture the BL21 strain which contained pBD024-tCas9-CIBN transformant under various concentration of arabinose induction for 16 hours in 30℃ environment. The induced concentrations are 4mM 10mM 15mM 15mM 15mM. To test the express result, we used Ha as first antibody and rabbit-anti in western blot. As the result showed in figure10, the best induced concentration is 15mM.</p>
<p></p>