

Medium and Buffers

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare the SOC medium.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.
*Source from 2015 iGEM Exeter
For nucleic acids DNA/RNA separation
- TAE buffer (Tris-acetate-EDTA)
- TBE buffer (Tris-borate-EDTA)
- LAB buffer (Lithium-acetate-borate)

- Prepare the solution by mixing the ingredients stated above.
If not using pre-mixed LB agar powder, prepare the materials as below:
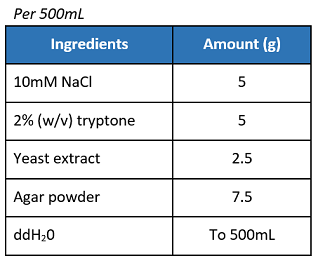
- In a 1L Erlenmeyer flask, swirl and mix the solution.
- Cover the top of the flask with a lid/aluminum foil and label with autoclave tape.
- Autoclave the liquid setting for 20 minutes or according to your autoclave's specifications.
- After removing the solution from the autoclave, allow the agar solution to cool to 55°C in an oven or water bath.
- When pouring the LB agar into plates, keep the bench area sterile by working near a flame or Bunsen burner. Alternatively, prepare the plates in a vacuum hood.
- Add the appropriate amount of desired antibiotic (refer the table below) to the solution and swirl to mix.
- Pour approximately 20mL of LB agar per 10cm polystyrene Petri dish.
- Place the lids on the plates and allow them to cool for until the agar is solidified.
- Label the bottom of plates with antibiotic and date before storing in plastic bags or sealed with Parafilm at 4°C.

Table source from New England Biolabs
Additional note:
- Antibiotic carbenicillin can be substituted for ampicillin in antibiotic selection plates [1].
If not using pre-mixed LB broth powder, prepare the materials as below:

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave.

- Prepare the solution by mixing the ingredients stated above.
- Sterilize in an autoclave before using it to prepare SOC medium.
Additional notes
- Some formulations of SOB use 10 mM MgCl2 and 10 mM MgSO4 instead of 20mM MgSO4.
- SOB medium is also available dry pre-mixed from Difco, 0443-17
- Adjust to pH 7.5 prior to use. This requires approximately 25mL of 1M NaOH per liter.
Source from OpenWetWare

- Prepare the solution by mixing the ingredients stated above.
- Filter sterilize using a 0.22 μm filter.
- Store at 4°C or -20°C.
*Source from OpenWetWare: TSS

Transformation
Ingredients:
Before starting the procedure, be sure to:
- Pre-chill centrifuge and cool rotor to 4°C
- Pre-chill 1L of 10% (w/v) glycerol solution on ice
- Pre-chill 500mL/1000mL Falcon tubes on ice
During the procedure:
Work under flame at all times.
- Grow a 5mL overnight culture of cells in LB media.
- In the morning, dilute this culture by at least 1/100 into a 50mL of fresh LB media in a 200mL conical flask.
- Incubate them at 37°C.
- Monitor growth of the cells every 30 minutes by measuring OD at 600nm (OD600) by filling up 750 µL of culture into cuvette. Use LB medium as blank.
- Once the cells are ready at OD600 = 0.4-0.6, harvest the cells to prepare for electroporation.
- Place cultures on ice for 15 minutes.
- Pour each 250mL culture into pre-chilled 500mL (or 1000mL) Falcon tubes.
- Centrifuge at 5000rpm for 10 minutes.
- Pour off the supernatant and aspirate any residual broth.
- Add 250 mL of pre-chilled 10% (w/v) glycerol to each of the centrifuge bottles and completely suspend the cells by pipetting the solution up and down.
- Centrifuge at 5000rpm for 10 minutes.
- Pour off the supernatant.
- Completely suspend the cells in 250mL glycerol and re-centrifuge at 5000rpm for 10 minutes.
- Pour off the supernatant and suspend the cells in the residual glycerol by pipetting up and down.
- At this point you can electroporate or freeze the cells away. To freeze, add 100µL of the culture to microcentrifuge tubes on ice.
- Once you have used all of the culture, transfer the tubes to dry ice for 10 minutes.
- Once the cultures are frozen, transfer them to a -80°C freezer. The cultures should be good for more than 6 months.
All subsequent steps should be carried out at 4°C.
Cells should be kept on ice wherever possible.
Ingredients:
Before starting the procedure, be sure to:
- warm SOC medium to 37°C
- Add 50µL of competent cells + 1 µL of DNA. Mix well and place on ice for 5 minutes.
- Transfer mix to 0.1cm electroporation cuvette (BioRad, #1652083)
- Perform electroporation in a MicroPulse Electroporator (BioRad) using one pulse of 1.80 kV (EC1).
- Measure the time constant (~5ms).
- Add 950µL of pre-warmed SOC medium (37°C) to the cuvette immediately after electroporation.
- Transfer to a fresh sterile 2mL tubes.
- Incubate at 37°C for 2 hours.
- Plate 50, 100, 200 µL (appropriate dilutions) and the rest of the cells (by centrifuging for 1 sec then decanting the liquid and resuspending with what is left in the tube) into LB agar plates using a plastic spreader.
- Incubate at 37°C overnight (approximately 16-18 hours).
- Look for transformants the next morning.
- Take note of the dilutions made to use for colony calculations next day.
*Source from New England Biolabs
Ingredients:
Before starting the procedure, be sure to:
- Pre-chill 1mL Eppendorf tubes
- Pre-chill TSS buffer
- Pre-chill Falcon tubes
During the procedure:
Work under flame at all times.
- Grow a 5mL overnight culture of cells in LB media.
- In the morning, dilute this culture by at least 1/100 into a 50mL of fresh LB media in a 200mL conical flask.
- Incubate them at 37°C.
- Monitor growth of the cells every 30 minutes by measuring OD at 600nm (OD600) by filling up 750 µL of culture into cuvette. Use LB medium as blank.
- Once the cells are ready at OD600 = 0.2-0.5, harvest the cells to prepare for chemical transformation.
- Split the culture into 50mL Falcon tubes and incubate on ice for 10 minutes.
- Centrifuge for 10 minutes at 3000 rpm and 4°C.
- Remove the supernatant. Pipette out any remaining media.
- Re-suspend in pre-chilled TSS buffer. The volume of TSS to use is 10% of the culture volume (i.e 25mL culture = 2.5mL of TSS to be added) that you spun down.
- Add 100µL aliquots to your chilled 1mL Eppendorf tubes and store at -80°C.
All subsequent steps should be carried out at 4°C.
Cells should be kept on ice wherever possible.
You may need to vortex gently to fully re-suspend the culture, keep an eye out for small cell aggregates even after the pellet is completely off the wall.
*Source from OpenWetWare
Ingredients:
Before starting the procedure, be sure to:
- Thaw competent cells from -80°C on ice
During the procedure:
Work under flame at all times.
- Add 50µL of competent cells + 1 µL of DNA (2.5µL of DNA if doing ligation).
- Mix well and place on ice for 30 minutes, or 5-10 minutes if in a rush.
- Heat shock the cells at 42°C for 30 seconds.
- Incubate on ice for 2 minutes.
- Add 450µL of SOC medium (kept in 4°C fridge) to the cells.
- Incubate for 45 minutes at 37°C in the shaker (30 minutes for Carbenicillin).
- Plate 50, 100, 200µL (appropriate dilutions) and the rest of the cells (by centrifuging for 1 second then decanting the liquid and resuspending with what is left in the tube) into antibiotic containing LB agar plates using a plastic spreader.
- Incubate at 37°C overnight (approximately 16-18 hours).
- Look for transformants the next morning.
*Source from OpenWetWare

Preparing overnight cultures
During the procedure:
Work under flame at all times.
- Prepare an overnight culture by inoculating one isolated single colony into 10mL of LB medium containing its respective antibiotic in a 50mL Falcon tube.
- Incubate in a shaker overnight at 37°C.

Bacterial glycerol stock
Ingredients
- 50% sterile glycerol
- Cryogenic vials
- Add 400µL of 50% glycerol to a cryogenic vial.
- Add 600µL of culture sample to be stored.
- Gently vortex the cryogenic vial to ensure the culture and glycerol is well-mixed. Alternatively, pipet to mix.
- On the side of the vial list all relevant information, including: Part, vector, strain, date, researcher etc.
- Store in a freezer box in a -80°C freezer.
Additional note:
- While it is possible to make a long term stock from cells in stationary phase, ideally your culture should be in logarithmic growth phase.
*Source from OpenWetWare

MINIPREP
Ingredients:
- Buffer P1
- Buffer P2
- Buffer N3
- Buffer PB
- Buffer PE
- Milli-Q water
Before starting the procedure, check if:
- Ethanol has been added to the PE and P1 buffer
- If added, there will be a tick on the lid.
- If not, add according to instructions labelled on the bottle.
- Spin down the overnight cultures at 10000 rpm at 4°C for 10 minutes using the communal centrifuge or 20mins at 4000rpm.
- Discard the supernatant. Bacteria will be pelleted at the bottom of the 50mL Falcon tube.
- Resuspend the pelleted bacteria with 250µL of Buffer P1 (always on ice).
- Transfer the re-suspended bacteria to a fresh 2mL Eppendorf tube.
- Add 250µL of Buffer P2 to the tube with bacteria and mix gently. Your sample should turn blue.
- Incubate for 5 minutes at room temperature
- Add 350µL of Buffer N3 and mix gently. Your sample should be colourless and should contain a white precipitant.
- Centrifuge samples at 14000 rpm for 10 minutes using a tabletop centrifuge.
- Transfer 750µL of the supernatant to a QIAprep Spin column (blue column).
- Centrifuge at 11000 rpm for 1 minute using a tabletop centrifuge.
- Pour the flow through again onto the column to increase the DNA concentration but do not close the cap tightly.
- Spin it down quickly for 3 seconds using the table top centrifuge/let it sit for at least 10 minutes. Discard the flow through.
- Add 500µL Buffer PB to the column.
- Centrifuge at 13000 rpm for 30 seconds using a tabletop centrifuge.
- Discard the flow through. Add 750µL Buffer PE to the column.
- Incubate at room temperature for 5 minutes.
- Centrifuge at 13000 rpm for 1 minute. Discard supernatant.
- Centrifuge at 13000 rpm for 1 minute.
- Transfer column to a fresh 1.5 mL Eppendorf tube.
- Add 30µL of Milli-Q water (use lesser amount if concentration before miniprep is low)
- Incubate for 5 minutes at room temperature.
- Centrifuge at 11000 rpm for 1 minute.
- Check concentration of DNA using Nanodrop.
(IMPORTANT: Do not allow more than 5 min incubation as this would degrade your plasmid)
*Source from Qiagen

















