Djcamenares (Talk | contribs) (Created page with " <html> <style> #kbcc ul { list-style-type: none; margin: 0; padding: 0; overflow: hidden; background-color: #333; } #kbcc li { float: left; } #kbcc...") |
Djcamenares (Talk | contribs) |
||
| Line 42: | Line 42: | ||
[[File:T--Kingsborough_NY--pathway_button.png|frame|left]] | [[File:T--Kingsborough_NY--pathway_button.png|frame|left]] | ||
| − | |||
| − | + | ||
| − | + | == Anaerobic Ammonia Oxidation == | |
| − | + | ||
| − | + | ||
Multistep conversion of ammonia into nitrogen gas, requiring several bacteria species, is not the only route for nitrogen to return to the atmosphere. Instead, anaerobic ammonia oxidation, also known as anammox, combines ammonia and nitrate to generate Nitrogen gas and water. Bacteria such as Kuenenia stuttgartiensis can carry out these reactions alone and without oxygen. In addition, these bacteria can derive metabolic energy from these reactions, which further reduces the need to supply an energy source. While these bacteria do not have the same demanding metabolic requirements as the microbial communities traditionally used, they present their own unique challenges for growth and application in an industrial setting. A few sewage treatment plants, such as the treatment center in Rotterdam (Netherlands), have successfully piloted the use of anammox bacteria. This success is despite difficulty cultivating and growing this particular species of bacteria. | Multistep conversion of ammonia into nitrogen gas, requiring several bacteria species, is not the only route for nitrogen to return to the atmosphere. Instead, anaerobic ammonia oxidation, also known as anammox, combines ammonia and nitrate to generate Nitrogen gas and water. Bacteria such as Kuenenia stuttgartiensis can carry out these reactions alone and without oxygen. In addition, these bacteria can derive metabolic energy from these reactions, which further reduces the need to supply an energy source. While these bacteria do not have the same demanding metabolic requirements as the microbial communities traditionally used, they present their own unique challenges for growth and application in an industrial setting. A few sewage treatment plants, such as the treatment center in Rotterdam (Netherlands), have successfully piloted the use of anammox bacteria. This success is despite difficulty cultivating and growing this particular species of bacteria. | ||
| − | |||
| − | + | == Our Project Goal == | |
| − | + | ||
| − | + | ||
| − | + | ||
The ultimate goal of the Kingsborough Community College team will be to import the enzymes needed for the anammox pathway into a more amenable microbial chassis, such as Escherichia coli. Other teams in the past have attempted to address the issue of biological nitrogen removal, but with limited success. The 2013 DTU Denmark team attempted to import traditional nitrifying and denitrifying enzymes from different species into E. coli. While they demonstrated a certain tolerance of E. coli to ammonia and nitrates, the altered metabolism of their engineered cell produced a toxic side effect. | The ultimate goal of the Kingsborough Community College team will be to import the enzymes needed for the anammox pathway into a more amenable microbial chassis, such as Escherichia coli. Other teams in the past have attempted to address the issue of biological nitrogen removal, but with limited success. The 2013 DTU Denmark team attempted to import traditional nitrifying and denitrifying enzymes from different species into E. coli. While they demonstrated a certain tolerance of E. coli to ammonia and nitrates, the altered metabolism of their engineered cell produced a toxic side effect. | ||
| − | + | ||
| − | + | ||
In contrast, the 2013 AHUT Chinese team attempted to import several different anammox components from K. stuttgartiensis. While this is a more desirable approach, they did not report any expression from their constructs. There are several, potentially solvable technical reasons why this result was obtained. | In contrast, the 2013 AHUT Chinese team attempted to import several different anammox components from K. stuttgartiensis. While this is a more desirable approach, they did not report any expression from their constructs. There are several, potentially solvable technical reasons why this result was obtained. | ||
| − | + | ||
| − | + | ||
The Kingsborough iGEM team will build upon the work previously done by both the Danish and Chinese teams. Instead of importing all the K. stuttgartiensis enzymes, we will focus only on successfully engineering a hydrazine synthase in E. coli. This enzyme is a key step in the anammox pathway, and it combines ammonia and nitric oxide to form hydrazine. Hydrazine is an intermediate towards nitrogen gas production, and it is useful on its own as a rocket fuel. Since E. coli can tolerate the presence of the substrates for this reaction, it will not be necessary to import all enzymes; in theory, the in vivo activity of hydrazine synthase can be tested and verified before trying to develop the complete anammox system. | The Kingsborough iGEM team will build upon the work previously done by both the Danish and Chinese teams. Instead of importing all the K. stuttgartiensis enzymes, we will focus only on successfully engineering a hydrazine synthase in E. coli. This enzyme is a key step in the anammox pathway, and it combines ammonia and nitric oxide to form hydrazine. Hydrazine is an intermediate towards nitrogen gas production, and it is useful on its own as a rocket fuel. Since E. coli can tolerate the presence of the substrates for this reaction, it will not be necessary to import all enzymes; in theory, the in vivo activity of hydrazine synthase can be tested and verified before trying to develop the complete anammox system. | ||
| − | + | ||
| − | + | ||
Recent work has identified the gene cluster encoding the hydrazine synthase complex. These genes will be codon optimized and cloned into E. coli under an inducible promoter. Growth of the modified bacteria in the presence of ammonia and nitric oxide will be monitored. After expression of the new cassette is activated, hydrazine production and concomitant hydrazine toxicity induced cell death should be observed. If time permits, this project will be expanded by identifying an ammonia or nitric oxide responsive promoter and incorporating it to control the expression of the hydrazine synthase. | Recent work has identified the gene cluster encoding the hydrazine synthase complex. These genes will be codon optimized and cloned into E. coli under an inducible promoter. Growth of the modified bacteria in the presence of ammonia and nitric oxide will be monitored. After expression of the new cassette is activated, hydrazine production and concomitant hydrazine toxicity induced cell death should be observed. If time permits, this project will be expanded by identifying an ammonia or nitric oxide responsive promoter and incorporating it to control the expression of the hydrazine synthase. | ||
| − | |||
| − | + | ||
| − | + | == Selected References == | |
| − | + | 1. Kartal, B., et al (2011) Molecular Mechanism of anaerobic ammonium oxidation. Nature. Oct 2; 479(7371) PMID: 21964329 | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
2. Russ, L, et al (2012) Genome analysis and heterologous expression of acetate-activating enzymes in the anammox bacterium Kuenenia stuttgartiensis. Arch Microbiology. Nov; 194(11) 943-8; PMID: 22752113 / PMC: 3477478 | 2. Russ, L, et al (2012) Genome analysis and heterologous expression of acetate-activating enzymes in the anammox bacterium Kuenenia stuttgartiensis. Arch Microbiology. Nov; 194(11) 943-8; PMID: 22752113 / PMC: 3477478 | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | 3. Knight, H. (2010) Bugs will give us free power while cleaning out sewage. NewScientist http://www.newscientist.com/article/dn18872-bugs-will-give-us-free-power-while-cleaning-our-sewage.html#.VMkPPWTF_ix | |
Revision as of 22:46, 2 November 2016
Anaerobic Ammonia Oxidation
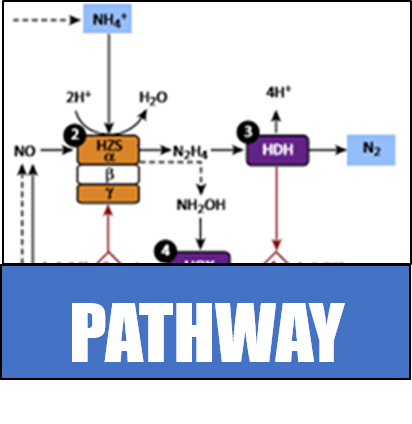
Multistep conversion of ammonia into nitrogen gas, requiring several bacteria species, is not the only route for nitrogen to return to the atmosphere. Instead, anaerobic ammonia oxidation, also known as anammox, combines ammonia and nitrate to generate Nitrogen gas and water. Bacteria such as Kuenenia stuttgartiensis can carry out these reactions alone and without oxygen. In addition, these bacteria can derive metabolic energy from these reactions, which further reduces the need to supply an energy source. While these bacteria do not have the same demanding metabolic requirements as the microbial communities traditionally used, they present their own unique challenges for growth and application in an industrial setting. A few sewage treatment plants, such as the treatment center in Rotterdam (Netherlands), have successfully piloted the use of anammox bacteria. This success is despite difficulty cultivating and growing this particular species of bacteria.
Our Project Goal
The ultimate goal of the Kingsborough Community College team will be to import the enzymes needed for the anammox pathway into a more amenable microbial chassis, such as Escherichia coli. Other teams in the past have attempted to address the issue of biological nitrogen removal, but with limited success. The 2013 DTU Denmark team attempted to import traditional nitrifying and denitrifying enzymes from different species into E. coli. While they demonstrated a certain tolerance of E. coli to ammonia and nitrates, the altered metabolism of their engineered cell produced a toxic side effect.
In contrast, the 2013 AHUT Chinese team attempted to import several different anammox components from K. stuttgartiensis. While this is a more desirable approach, they did not report any expression from their constructs. There are several, potentially solvable technical reasons why this result was obtained.
The Kingsborough iGEM team will build upon the work previously done by both the Danish and Chinese teams. Instead of importing all the K. stuttgartiensis enzymes, we will focus only on successfully engineering a hydrazine synthase in E. coli. This enzyme is a key step in the anammox pathway, and it combines ammonia and nitric oxide to form hydrazine. Hydrazine is an intermediate towards nitrogen gas production, and it is useful on its own as a rocket fuel. Since E. coli can tolerate the presence of the substrates for this reaction, it will not be necessary to import all enzymes; in theory, the in vivo activity of hydrazine synthase can be tested and verified before trying to develop the complete anammox system.
Recent work has identified the gene cluster encoding the hydrazine synthase complex. These genes will be codon optimized and cloned into E. coli under an inducible promoter. Growth of the modified bacteria in the presence of ammonia and nitric oxide will be monitored. After expression of the new cassette is activated, hydrazine production and concomitant hydrazine toxicity induced cell death should be observed. If time permits, this project will be expanded by identifying an ammonia or nitric oxide responsive promoter and incorporating it to control the expression of the hydrazine synthase.
Selected References
1. Kartal, B., et al (2011) Molecular Mechanism of anaerobic ammonium oxidation. Nature. Oct 2; 479(7371) PMID: 21964329
2. Russ, L, et al (2012) Genome analysis and heterologous expression of acetate-activating enzymes in the anammox bacterium Kuenenia stuttgartiensis. Arch Microbiology. Nov; 194(11) 943-8; PMID: 22752113 / PMC: 3477478
3. Knight, H. (2010) Bugs will give us free power while cleaning out sewage. NewScientist http://www.newscientist.com/article/dn18872-bugs-will-give-us-free-power-while-cleaning-our-sewage.html#.VMkPPWTF_ix