Result
Cell Cycle Dependent Promoter: Pnrd
As described in the systems page, a cell division dependent promoter is essential for the functioning of our intergenerational phenotypic switching device. Stanford-Brown iGEM 2012 documented the characterisation of Promoter Pnrd ([http://parts.igem.org/Part:BBa_K847211 BBa_K847211])
and confirmed its functionality. However, since it was submitted in RFC[25] standard, the part was redesigned in RFC[10] to deal with the possible disadvantages of RFC[25] standard.
Sequencing results of Pnrd RFC[10]
The results of sequence show that the improved part contains the RFC[10] prefix and suffix.
Reconstructed in the RFC[10] format, the Pnrd promoter was ligated in front of part BBa_E0240 and compared to the standard promoter BBa_J23101. The measurement was made approximately 4h after 100 fold dilution in M9 in a 5 min interval. For details of our characterisation, refer to our protocol.
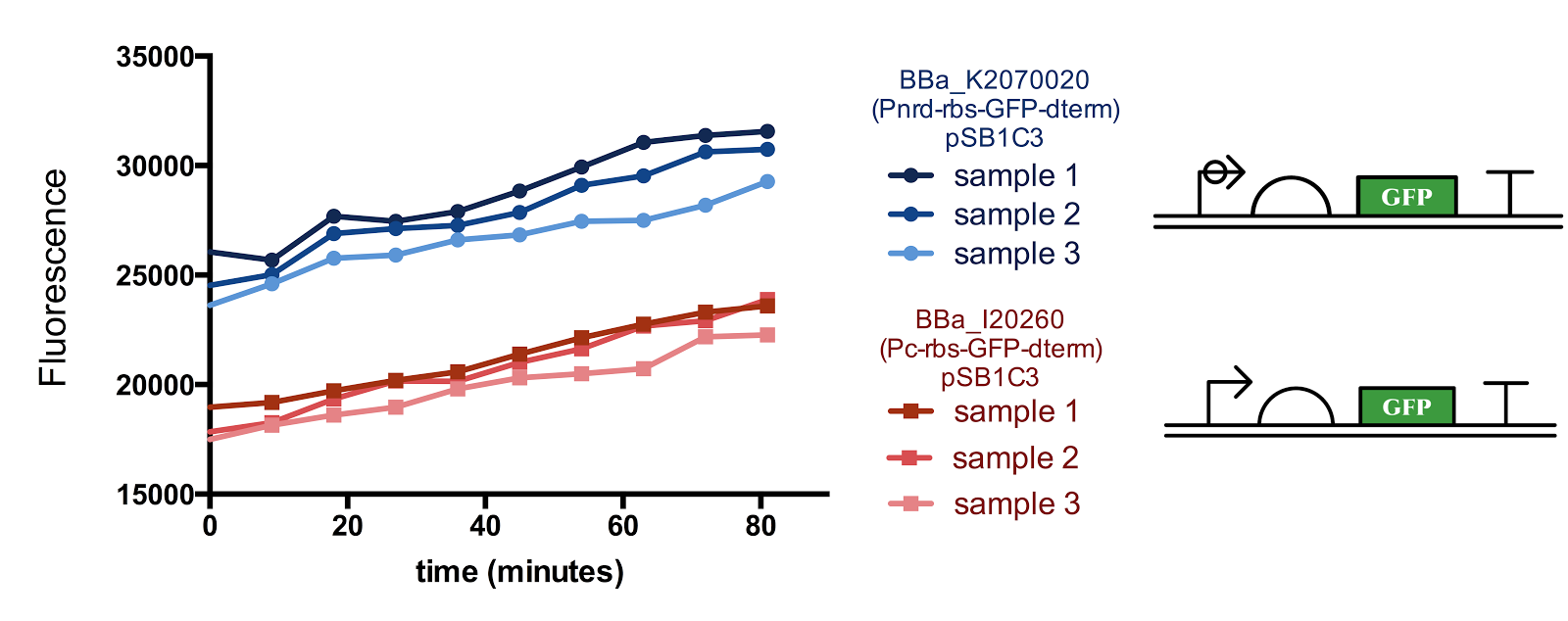
Figure 1. Measurement of nrd Promoter Activity.
The fluorescence from the Pnrd promoter is compared with that from the standard promoter BBa_J23101, 3 samples were measured every 9 minutes for each construct. Although there seems to be a slight sudden rise in the promoter activity from the 10-20th minute after the start of measurement, since the change in cell concentration could not be measured simultaneously with fluorescence, it is not certain whether the rise is influenced by the cell cycle/division.
SIGMA 35
In addition to the cell division dependent promoter, extracytoplasmic function (ECF) sigma factors are required in our device to coordinate between the cell division dependent promoter and Toehold Switch.
The figure below shows the result of our characterisation. Experiments were done according to methods described in our protocol.
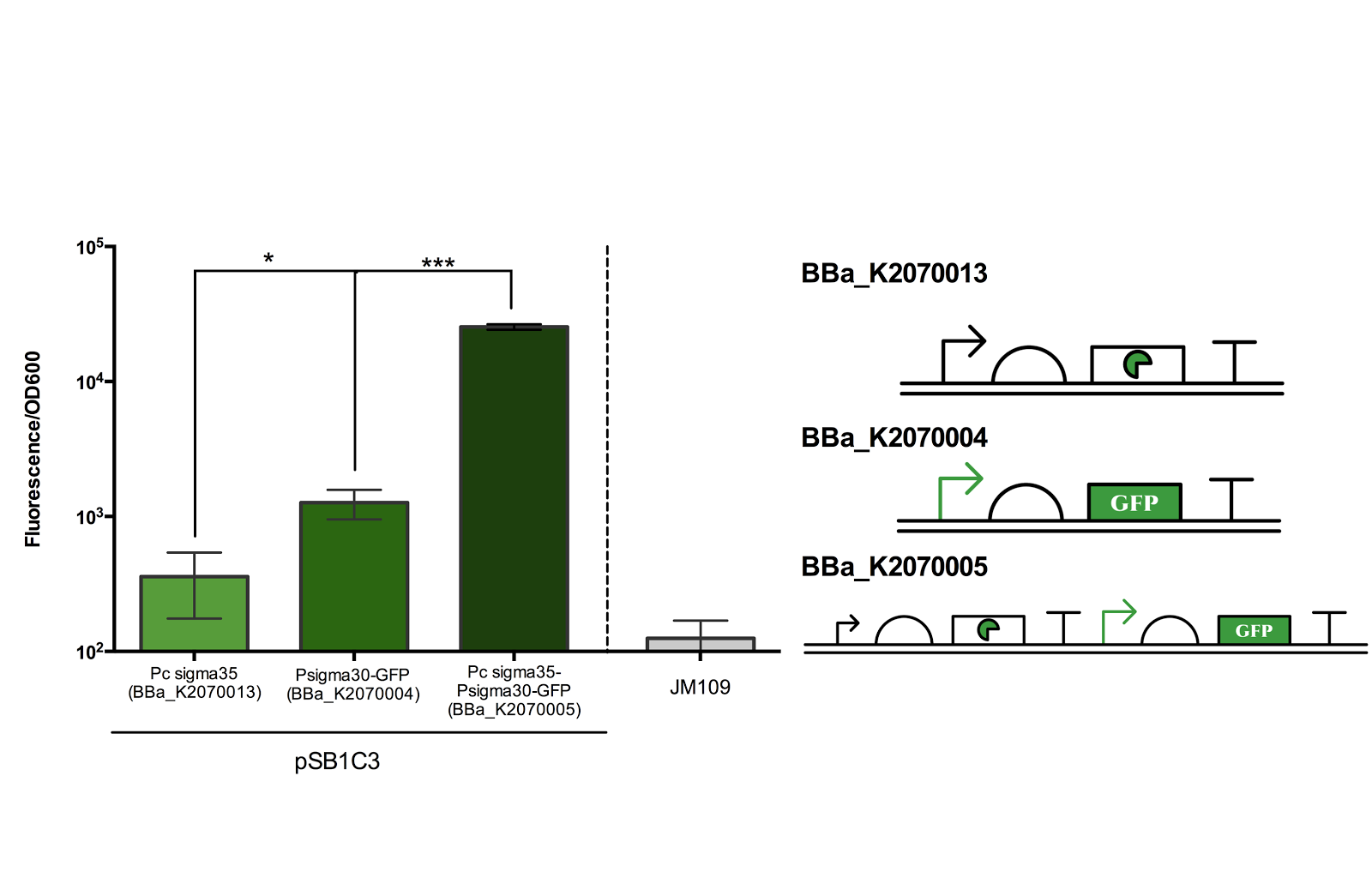
Figure 2. Measurement of ECF sigma35 and sigma30 promoter activity.
Fluorescence/OD600 of a construct containing both an ECF sigma35 generating device and ECF sigma 35 inducible GFP generating device BBa_K2070005 was compared to that containing only either one of the devices. *** for P> 0.001 indicates an extremely significant difference between the induced and uninduced state.
As can be seen from the above figure, the ECF sigma30 promoter is only activated in presence of ECF sigma35, suggesting that that the parts and devices constructed worked as expected. The ON state, measured in Fluorescence/OD600, was up to more than 10 fold of the OFF state. However, it should be noted that there is a leak in the promoter in its OFF state as indicated by the significance difference in Fluorescence/OD600 when compared to the construct expressing only sigma 35.
Once the functionality of the promoter and its cognate pair was confirmed, the part was ligated in pSB3K3 to measure its strength relative to the standard promoter BBa_J23101. The strength of the ECF30 promoter in its induced state was measured to be of RPU 3.2 units.
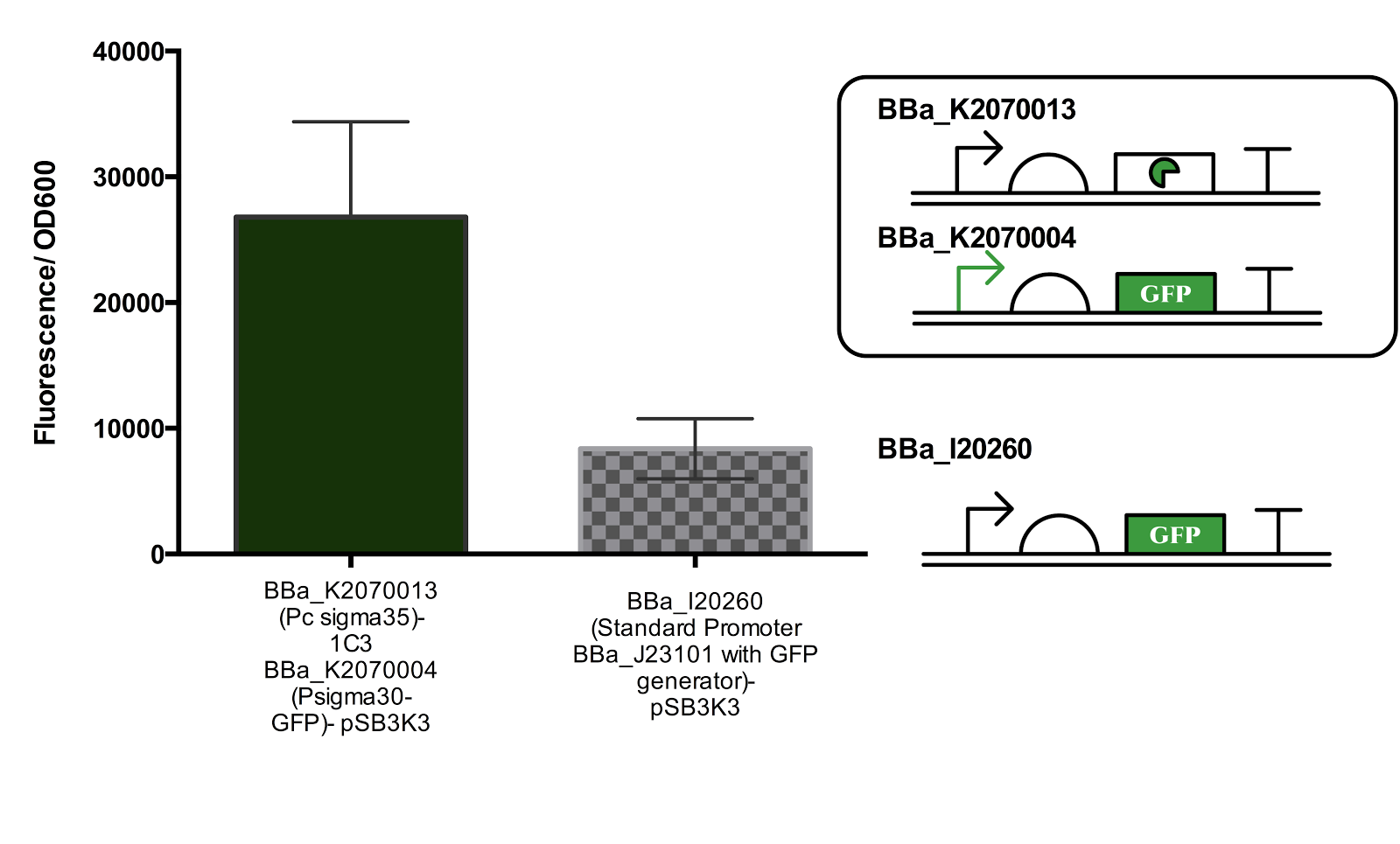
Figure 3. Measurement of Activated Sigma30 promoter Activity in comparison to Promoter BBa_J23101.
Both parts are ligated in plasmid pSB3K3. The part containing the constitutive sigma35 generator was double transformed into the cell for measurement. Data represents an average of 3 samples and error bars represent the standard deviation.
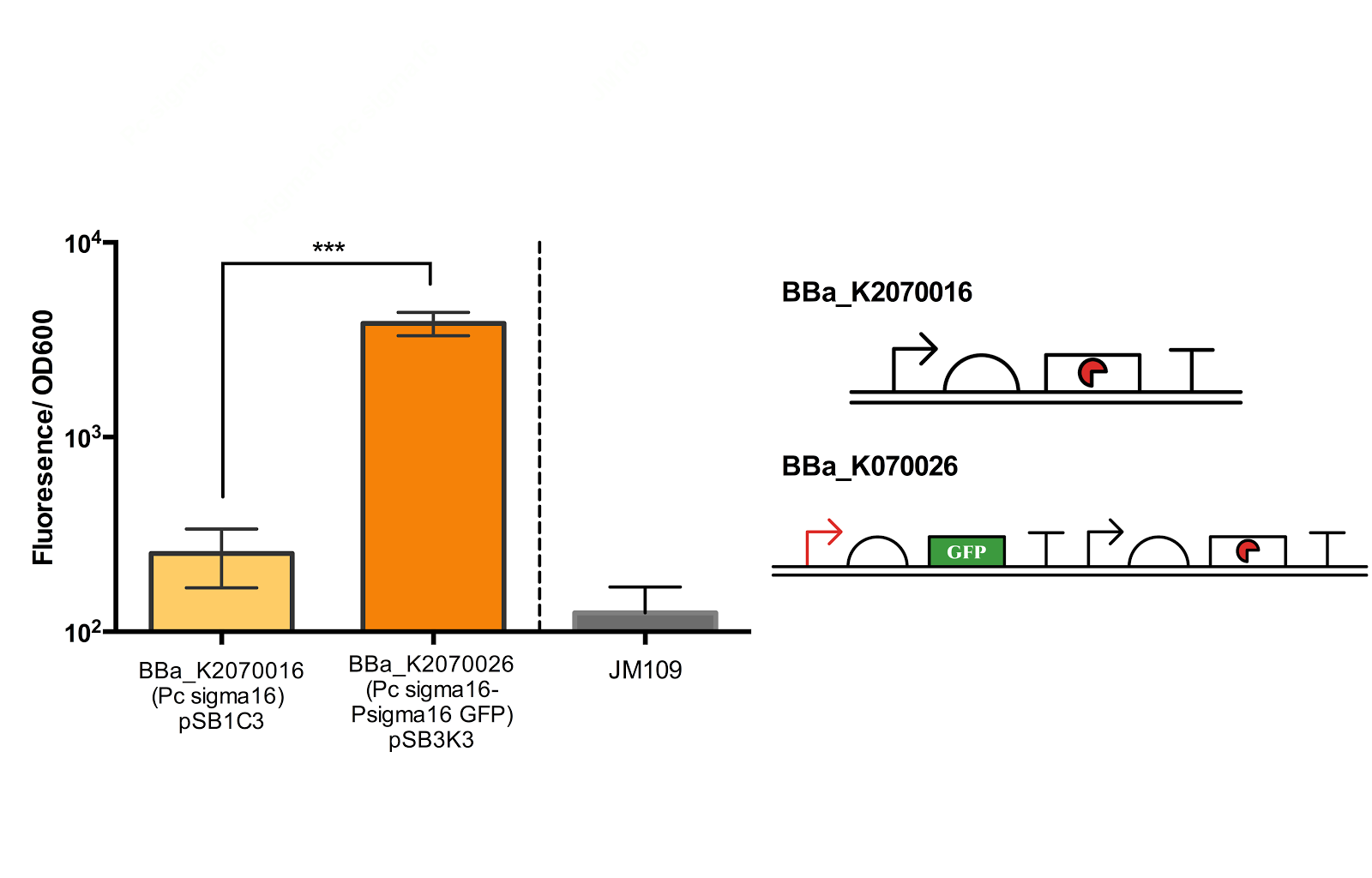
SIGMA 16
The second sigma factor that we have characterised and tested its functionality is ECF sigma16 from Pseudomonas entomophila.
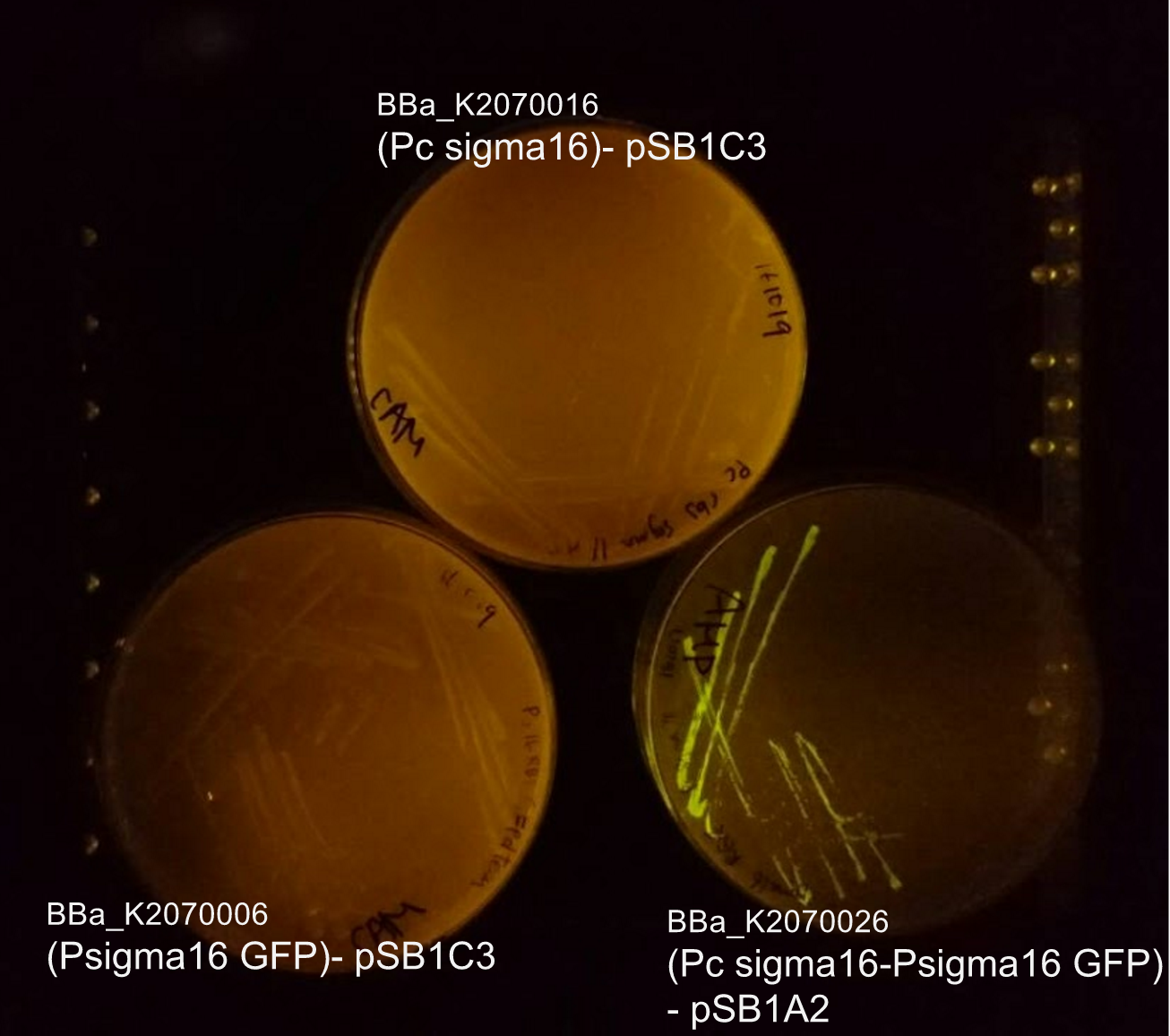
From bottom left: BBa_K2070006 (ECF sigma16 inducible GFP reporter), BBa_K2070026 (Constitutive ecf16 generator with ecf 16 inducible GFP reporter), BBa_K2070016 (Constitutive ECF sigma16 generator). As only cells with BBa_K2070026 is shown to fluorescence, it can be suggested that the devices works as expected.
Though we have checked its functionality on agar plate, due to time constraints, we were not able to measure the fluorescence of the Psigma16 GFP (BBa_using the spectrofluorophotometer.
Figure 5. Measurement of ECF sigma16 and Psigma16 GFP promoter activity.
Fluorescence/OD600 of a construct containing both an ECF sigma16 generating device and ECF sigma 16 inducible GFP and construct containing only the sigma 16 generator was measured. Measurement results indicate that there is a extremely significant difference between the two groups. Data taken from the average of 3 samples, with error bars indicating the standard deviation.
Though the fluorescence measurement results indicate that there is a extremely significant difference between the construct BBa_K2070016 and BBa_K2070026, since we were not able to compare the measurement with Psigma 16 GFP, we cannot be sure how much of the fluorescence is due to the activation of the sigma promoter and not the leak from the inducible promoter. Thus, further measurement is necessary to better confirm the activity of the ECF sigma16 and its cognate promoter.
SIGMA 11
ECF sigma 11 ([http://parts.igem.org/Part:BBa_K1461005 BBa_K1461005]) and its orthogonal sigma11 promoter([http://parts.igem.org/Part:BBa_K1461002 BBa_K1461002]) was characterised by Team UT-Tokyo in 2014. Since our system in design requires 3 ECF sigma factors and their corresponding promoter, in addition to the new sigma factors that we constructed, we decided to improve the characterisation of the existing the ECF sigma parts so that we can better understand their activity for the use in our system.
According to our modelling results, the concentration of sigma factors must be tuned so that while it must be high enough to activate the sigma promoter activity, it should not be too high that it cannot be repressed by anti sigma factors.
The approach that was taken in 2014 to tune the expression of ECF sigma factors was to tune the promoter that transcribes the genes coding for the ECF sigma factors. However, they reported that due to the possible leak from the PBAD promoter, the sigma expression could not be tuned appropriately.
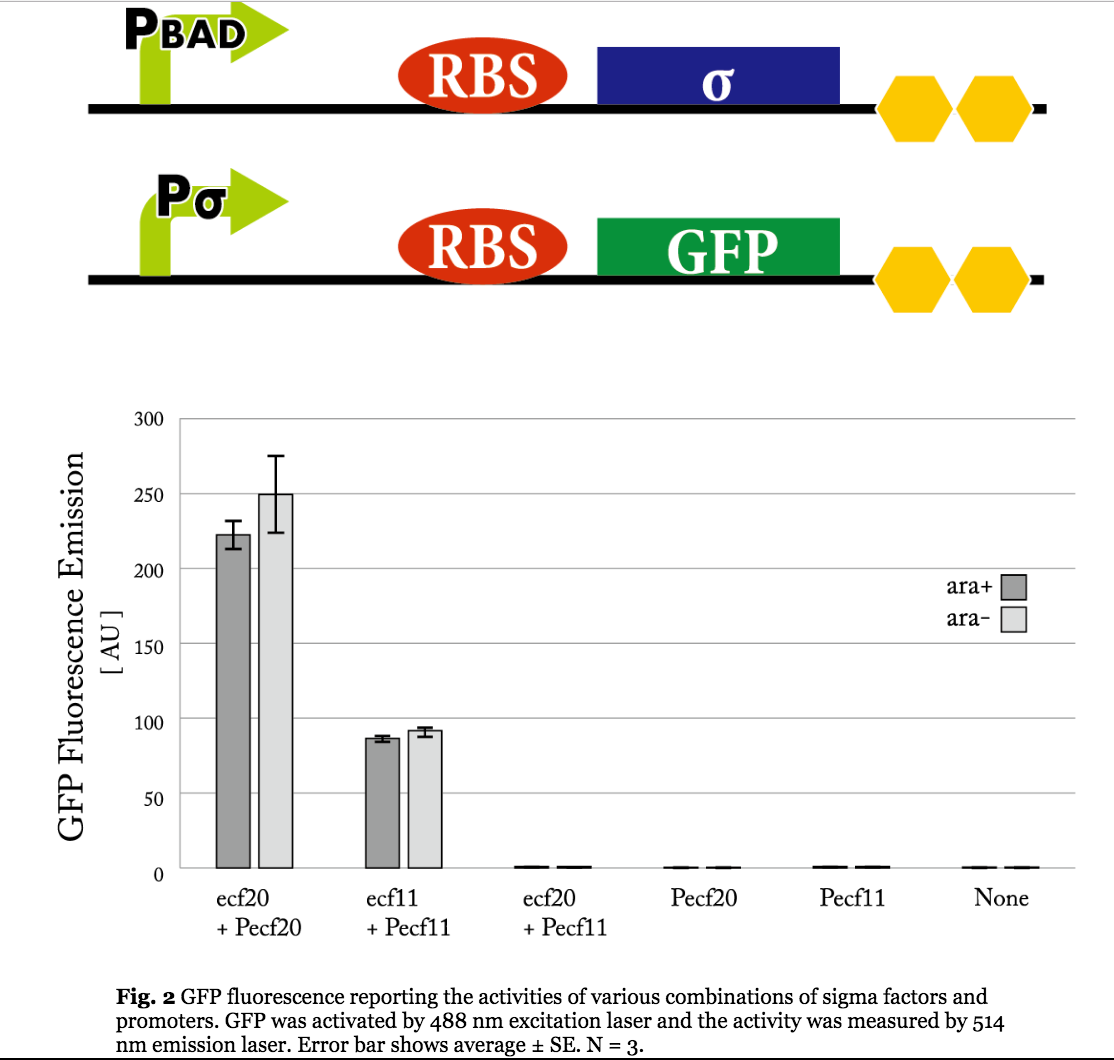
Figure 5. Previous iGEM results of sigma 11 activity. (Taken from UT-Tokyo 2014 wiki.)
Results indicate that though the ECF sigma11 factors were functional in activating the corresponding promoters, they were not able to influence the level of activation by tuning the expression level of promoter generating sigma factors.
We thus decided to test constitutive promoters of different strengths for generating the sigma factors and as well take another approach to tuning the induction of the sigma promoters, that is, by expressing the sigma factors on a plasmids of different copy number.
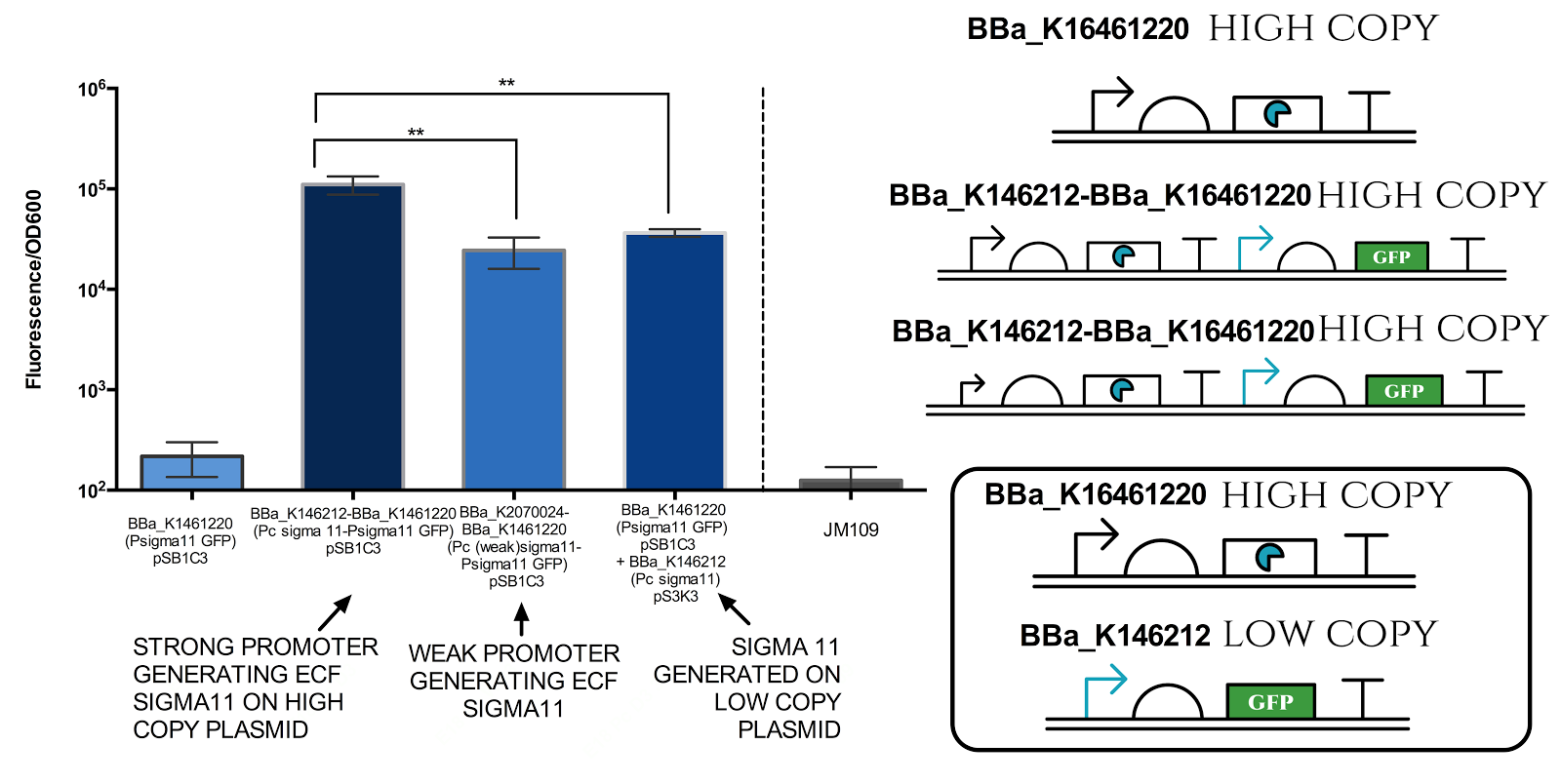
Figure 6. Measurement of sigma 11 promoter activity under different sigma11 generating conditions.
Strong promoter refers to BBa_J23101, while weak promoter refers to BBa_J23109. High copy plasmid refers to pSB1C3 (copy number of 100-300 per cell) and low copy plasmid refers to pSB3K3 (copy number of 10-12 per cell). Graph illustrates that there is a very significant difference( ** indicates P value 0.001 to 0.01) between different conditions. Data represents an average of 3 samples. Error bars show standard deviation.
The results above indicate that it is possible to tune the expression of the sigma 11 promoter by tuning levels of sigma11. The first approach is to change the expression level of promoter generating the sigma factors, and the second approach is to change the copy number of plasmid in which the sigma factors are expressed.
Anti Sigma Factors
To be used together with the ECF sigma11 and its promoter pair is anti sigma11 ([http://parts.igem.org/Part:BBa_K1461007 BBa_K1461007]) reported to be able to repress the activity of ECF sigma 11 in activating the sigma11 promoter. However, when its functionality was tested previously, by iGEM-UT Tokyo 2014, the results suggested that the anti sigma 11 was not able to repress the activity of ECF 11. It was hypothesised that anti sigma11 not functioning due to “ the sigma factors [were] expressed more than the anti-sigma factors could repress their activation of the corresponding promoters.” Thus, we attempted a new characterisation this time ligating anti 11 on a high copy plasmid and ECF sigma11 on a low copy plasmid in order to increase the level of anti 11 relative to sigma11.
The figures below show the result of our characterisation and the previous characterisation of anti 11 done by UT-Tokyo 2014.
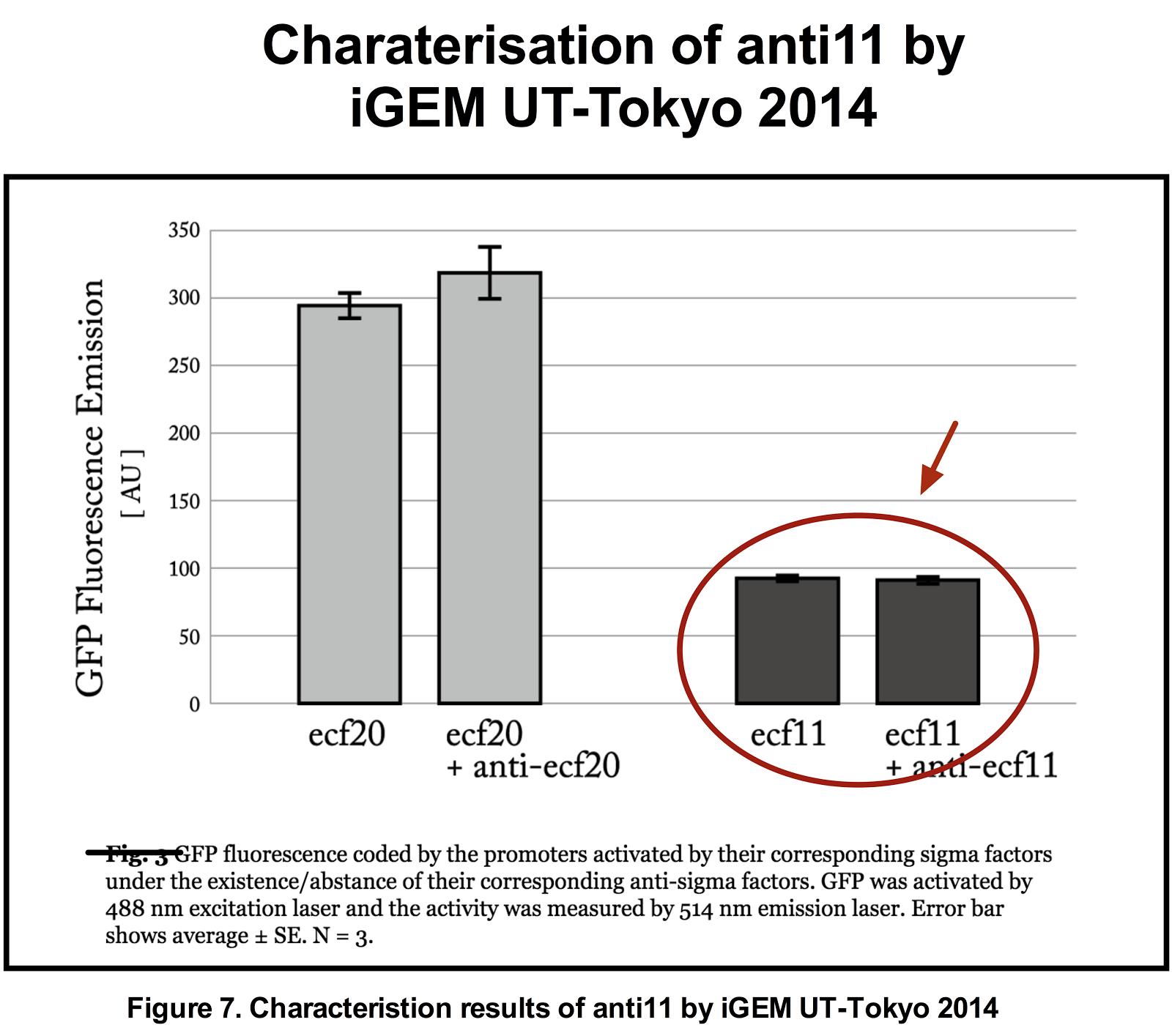
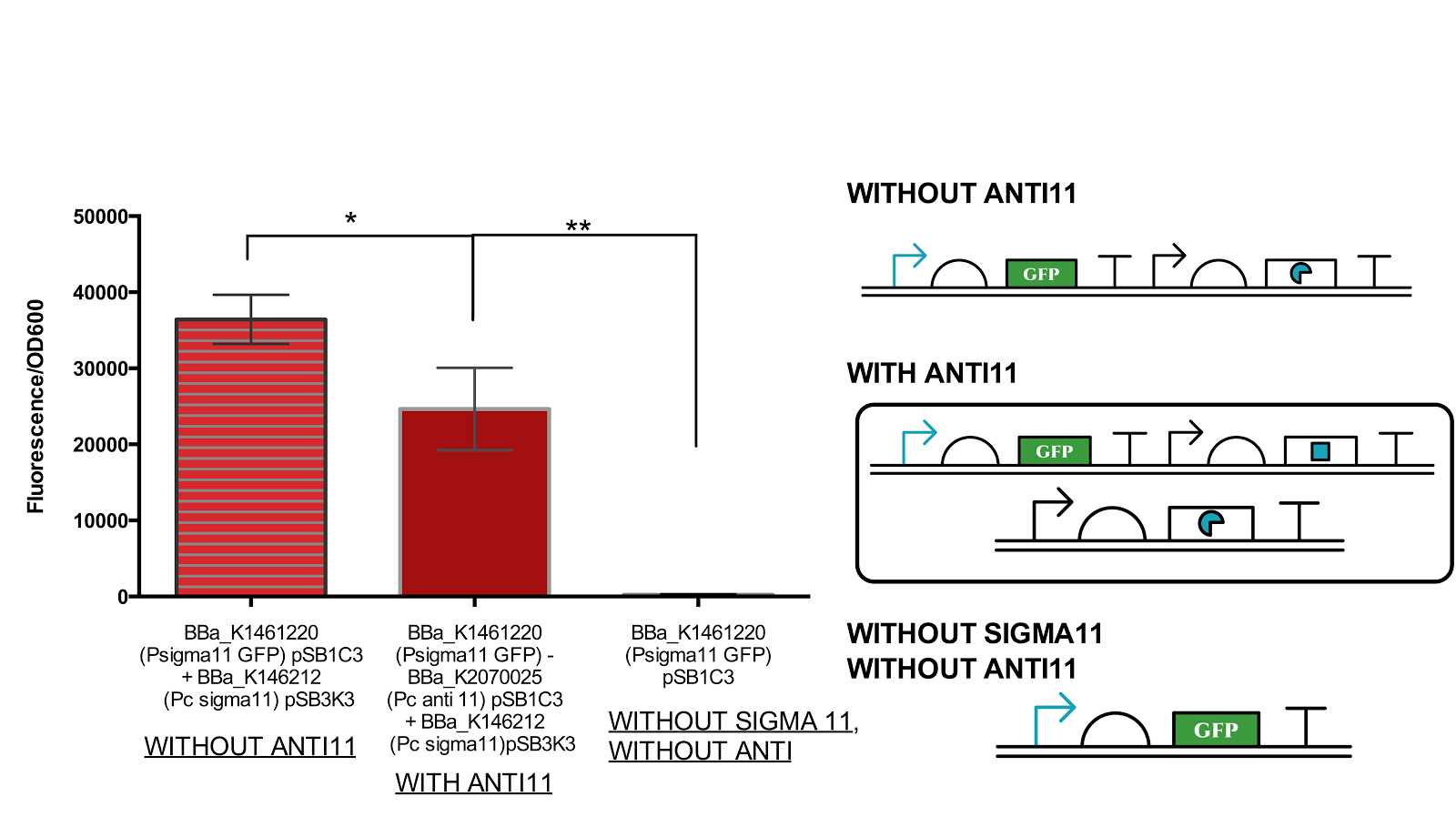
Figure 8. Functionality assay of anti 11 by increasing concentration of anti11 relative to sigma11.
Instead of generating sigma11 on backbone pSB1C3 as done in by iGEM UT-Tokyo 2014, sigma 11 was generated on backbone pSB3K3 and anti11 in pSB1C3 as an attempted to increase the level of anti11 relative to sigma11. The measurement shows that there is a significant level of difference between the anti sigma 11(-) construct and antisigma 11 (+). However, the difference between the complete OFF state (without sigma) and with anti 11 state is also very significant. Data represents an average of 3 samples with error bars representing the standard deviation.
From figure 6 above, it can be suggested that anti sigma11 can function to inhibit the activity of sigma11 as the fluorescence output from the sigma 11 promoter was significantly reduced in presence of anti11. Nevertheless, the anti sigma11 is not strong enough to function as a “switch” that can turn the promoter back to the state when there is no ECF sigma11 present.
D. The Rainbow
Completing the assay for the day, we obtained a beautiful piece of art on our screen.
Figure 9. Niji.
The art piece is named Niji, meaning rainbow in Japanese. Each line of the Niji represents a step closer to the results that we obtained.