| Line 17: | Line 17: | ||
</div> | </div> | ||
</div> | </div> | ||
| − | <p>All Senders and Receivers were gel verified and sequenced with the help of the Arizona State University's Core Laboratory. After confirmation of the successful construction of each part, each part was retransformed to create sufficient stocks for later experiments. In addition, the | + | <p>All Senders and Receivers were gel verified and sequenced with the help of the Arizona State University's Core Laboratory. After confirmation of the successful construction of each part, each part was retransformed to create sufficient stocks for later experiments. In addition, the 5 Senders that were submitted to the registry were taken out of their respective modular vectors and placed into psB1C3 Biobrick standard vector. The Modular Sender Vector was submitted within its own backbone, as it was designed with an RFC10 prefix within the part.</p> |
<h2>Inducer Confirmation through Optical Density Measurements</h2> | <h2>Inducer Confirmation through Optical Density Measurements</h2> | ||
<p>Initially, after the construction of the 20 Receivers and Senders, the focus of our project centered on the Aub system. This was due to the highly uncharacterized nature of the Aub system as well as possible discoveries of orthogonality between Aub and other system receivers. However, we encountered difficulties with the AubR transcription factor, which would not be highly induced in the presence of the Aub AHL. We shifted our focus to the inducers, seeking to create a part collection of 5 novel inducers that were previously unavailable in the iGEM registry. While we had successfully sequenced each of the constructed Senders, a proof-of-concept test was conducted on the inducers to demonstrate the likely production of the AHL. </p> | <p>Initially, after the construction of the 20 Receivers and Senders, the focus of our project centered on the Aub system. This was due to the highly uncharacterized nature of the Aub system as well as possible discoveries of orthogonality between Aub and other system receivers. However, we encountered difficulties with the AubR transcription factor, which would not be highly induced in the presence of the Aub AHL. We shifted our focus to the inducers, seeking to create a part collection of 5 novel inducers that were previously unavailable in the iGEM registry. While we had successfully sequenced each of the constructed Senders, a proof-of-concept test was conducted on the inducers to demonstrate the likely production of the AHL. </p> | ||
Revision as of 11:51, 16 October 2016
Results
Introduction
The expanded characterization of N-acyl homoserine lactone (AHL) systems focused on 10 main systems, which are tabulated in the Project Description. The synthetic system that our team aimed to construct split the system into a Sender (AHL synthase) and a Receiver. By separating the inducer of the AHL from the receptor, crosstalk can be determined between systems.
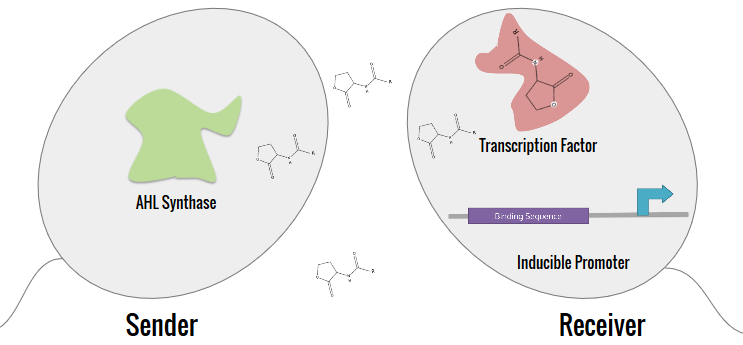
Bacterial Cloning
E. coli DH5αT was used for the construction of the Senders and Receivers. BL21(DE3) E. coli was used for induction tests. After long transformation, liquid cultures were made using LB broth mixed with ampicillin. All cultures were then appropriately digested and ligated in order to properly construct each part. A modular receiver and modular sender vectors were developed for each of the parts. The designs for these plasmids are shown below.


All Senders and Receivers were gel verified and sequenced with the help of the Arizona State University's Core Laboratory. After confirmation of the successful construction of each part, each part was retransformed to create sufficient stocks for later experiments. In addition, the 5 Senders that were submitted to the registry were taken out of their respective modular vectors and placed into psB1C3 Biobrick standard vector. The Modular Sender Vector was submitted within its own backbone, as it was designed with an RFC10 prefix within the part.
Inducer Confirmation through Optical Density Measurements
Initially, after the construction of the 20 Receivers and Senders, the focus of our project centered on the Aub system. This was due to the highly uncharacterized nature of the Aub system as well as possible discoveries of orthogonality between Aub and other system receivers. However, we encountered difficulties with the AubR transcription factor, which would not be highly induced in the presence of the Aub AHL. We shifted our focus to the inducers, seeking to create a part collection of 5 novel inducers that were previously unavailable in the iGEM registry. While we had successfully sequenced each of the constructed Senders, a proof-of-concept test was conducted on the inducers to demonstrate the likely production of the AHL.
The AHL gene lies behind a supposedly constitutive Tet promoter as well as an mCherry gene. If mCherry is produced, this will be a likely indicator that the AHL is also being produced. Each inducer was re-transformed into BL21(DE3) E. coli and then the liquid culture was plated on a 96-well plate in triplicate. An 8-hour optical density test was conducted on the 96-well plate, measuring at a wavelength of 600nm to demonstrate overall cellular growth and between 580-610nm to measure mCherry fluorescence. The growth curves of all ten systems are shown below:


Figure on left is growth curve over time measured at 600nm. Figure on right is growth curve over time measured at 580-610nm.
The figures above demonstrate that when the AHL synthase gene is grown in E. coli, the AHL is likely produced. The OD600 measurements affirm that bacterial growth occurred over the 8-hour read. All 10 systems produced mCherry, with 9/10 systems showing a positive growth trend over time. The 10th system, RpaI, had a negative trend in mCherry presence but also had, by far, the highest overall absorbance of mCherry. It is likely that there is a threshold to the mCherry production, which was reached by the Rpa system. These results provide a proof-of-concept that the Senders are able to produce AHLs in our selected chassis, as mCherry lies downstream of the Sender in our system, so the AHL is likely produced.
Mass Spectrometry
In order to confirm that the AHL is being produced within our chassis from the constructed Senders, mass spectrometry measurements were taken. The AHL samples were compared against a negative control, and a mass-charge ratio that matches that of the predicted AHL confirmed the production of AHLs by E. coli. First, a negative control was measured using a Dibutyl phthalate (DBP) matrix that would be used in the AHL measurements.


The AHL produced by the Lux/Esa systems (which is the same) was measured first, and produced peaks not found in the negative control at 85.6286 m/z and 128.5915 m/z. These values sum to the mass-charge ratio of the AHL produced by the Lux/Esa system. After further analysis, it was determined that these two peaks correspond to the lactone ring and the acyl tail of the AHL. It is likely that the matrix that was used reacted with the unstable carbon-nitrogen bond within the AHL, causing a fracture in the molecules. These results do suggest that the AHL was produced, but the production of a fully-formed molecule is not shown by this figure.
Matrix-assisted laser desorption/ionization (MALDI) was used to treat the AHL solution in order to prevent breakdown of the AHL molecules. The Aub system was chosen for this run, which is highly uncharacterized. After running mass spectrometry on the sample, the figure below was produced.


A peak at 283.9047 m/z was produced, which matches the mass-charge ratio predicted for the AubI AHL. This shows that the AHL was successfully produced by the AubI Sender gene inserted into E. coli. Due to time limitations, we were unable to complete mass spectrometry on the other systems that we constructed Senders for.
F2620 Induction Tests
Once the production of AHLs by the Senders was tested via the OD test and mass spectrometry, the interaction between the Senders and the well-characterized Bba_F2620 by Barry Canton and Anna Labno from MIT was tested. This system originates from the Lux system and is well-understood, which will likely provide the most immediate utility. This test will aim to determine orthogonality between the Senders and F2620 while also providing a direct comparison between systems' inductive capabilities.
The Induction test was done by extracting AHLs from the supernatant of a liquid culture and then plating with uninduced cells. This was done at a 10% and 50% AHL concentration, as well as 3 negative controls and 1 positive control. All samples were done in triplicate. The setup is shown below:

Each system generated its own curve, comparing the 10% and 50% AHL concentrations with a F2620 control curve. All ten growth curves are shown below:


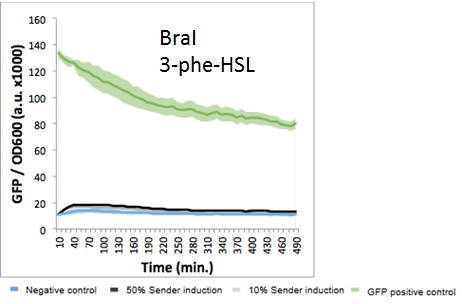









The figures above demonstrate a mix of orthogonal and intersecting(crosstalk) networks. Of the 10 systems measured, known crosstalk partners such as Esa, Las, and Rpa showed increased levels of GFP measured as compared to the F2620 control. Lux, the native system that F2620 was designed for, Las, Rpa, and surprisingly Cer showed an exponential increase in GFP absorbance with an increasing concentration, with much higher levels of absorbance at the 50% AHL concentration as compared to the 10% AHL concentration. Overall, Cer, Esa, Las, Lux, and Rpa showed a significant increase in GFP absorbance over the F2620 control, indicating some degree of crosstalk with F2620.
Meanwhile, the Aub, Bja, Bra, Rhl, and Sin systems showed evidence of orthogonality to F2620. All 5 systems maintained GFP absorbance levels near the F2620 control. BraI showed a slight increase in absorbance, making it difficult to conclude the level of interaction between the BraI AHL and F2620. SinI had lower absorbance levels in the sample runs than the F2620 control, but still remains in the margin of error for the plate reader. From these results, Aub, Bja, and Rhl are the strongest candidates to be orthogonal to F2620, with both AHL curves consistently close to control absorbance levels.

