| Line 77: | Line 77: | ||
<h4>Results</h4> | <h4>Results</h4> | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<h2>Construction</h2> | <h2>Construction</h2> | ||
| Line 246: | Line 89: | ||
manner.</p> | manner.</p> | ||
</div> | </div> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<table cellspacing="0" cellpadding="0"> | <table cellspacing="0" cellpadding="0"> | ||
Revision as of 11:58, 8 October 2016
Proof of Concept
Electrically Induced 'Light Bulb'
We plan to clone Escherichia coli so that it fluoresces when a current of around 400mA is passed through the broth that the E. coli are growing in.
Previous attempts at this experiment have used GFP to fluoresce with the current. In terms of this experiment we shall use GFP to ensure the design construct works. We have incorporated restriction sites within the construct so that we are able to then switch the GFP with other constructs, such as luciferase to emit light like an actual lightbulb.
In order for the GFP to be activated by a current, a heat-shock induced promoter must be present. Previous experiments have used sigma 32 as a heat shock promoter which seems to have the desired effect, although we shall test this in the lab. We have also inserted two restriction sites to allow us to either cut out the sigma 32 promoter, or add an extra promoter. This will allow us to test the effect that the sigma 32 has on the GFP production and whether it is induced by heat-shock or not.
The design has a natural ribosome binding site, which we will be adding to the registry (BBa_K1895001). This is to ensure that the ribosome does bind to the DNA and synthesise the protein correctly, however we will make variants of this DNA with two different medium bicistronic rbs. The medium bicistronic rbs will avoid the problem of placing too high a translational burden on the cell. We will then test all three variants to determine which is the best rbs to use in the final design.
Results


Arabinose Controlled 'Variable Resistor'
We plan to engineer Escherichia coli to behave like a variable resistor. We aim to do this by using E. coli to vary the amount of free ions in an electrolyte. Ion uptake will be controlled by the expression of smtA. SmtA is a metallothionein that can bind to heavy metal ions like cadmium (II), Zinc (II) and Copper (II).
SmtA has been used in a number of iGEM projects and is in the registry (BBa_K519010). It has previously been used in experiments for Cadmium (II) uptake, see Tokyo-NokoGen 2011. We will be examining firstly, the impact of smtA of Zinc (II) concertation rather than Cadmium (II) and then the impact that this has on the resistivity of the Zinc (II) containing media. In this instance we will be using Zinc sulfate (ZnSO4) in solution where it disassociates into Zn2+ and SO42- ions. Various concentrations of Zinc sulfate have known electrical conductivity . When smtA is expressed it will render the Zn2+ unavailable and thereby reduce the conductivity of the solution.
We will be placing smtA under the control of an AraC regulated promoter allowing the expression of smtA to be controlled by the addition or removal of arabinose.
Results
Construction
BioBrick Assembly
The fusion protein cph8 (BBa_I15010) is not available in the distribution kit, it is however in stock at the registry and can be ordered. The remaining parts are in the distribution so BioBrick assembly can be performed. However because of the large number of parts, and there are no intermediaries in the registry or distribution kit, this construct would be unwieldy to assemble in this manner.
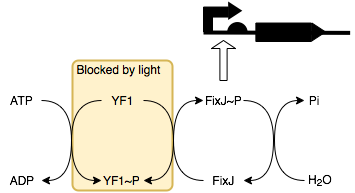 |
We will be placing smtA under the control of a FixJ-P (phosphorylated FixJ)
promoter. This allows it to be regulated by blue light through a series of
reactions with its response regulator protein YF1 (below).
In the absence of light, YF1 undergoes autophosphorylation to produce YF1-P which can then phosphorylate FixJ. This in turn activates the transcription of the downstream protein, in this case it is SmtA. Thus, in the presence of light SmtA is not produced and so conductivity does not change, whilst in the absence of light SmtA is produced resulting in a decrease in resistance.
Clearly, this behaviour is the inverse of an electrical light dependent resistor where resistance increases with light intensity. To mimic this behaviour using biological circuits we would place an inverter before the FixK2 promoter (which is activated by FixJ-P). The inverter is constructed by placing the desired output, here SmtA, under the control of a lambda cl regulated promoter (BBa_R0051). As lambda cl represses the promoter having this produced under control of FixK2 promoter inverts the system so that SmtA is produced in the presence of light rather than the absence thereof. BBa_K592020 is an example of a part that uses this technique.
Our Construct
The non-inverted construct is shown in Figure 1 and the inverted construct in Figure 2. Currently our device is shown as a 1 plasmid system but there is no reason that the two separate sub-components could not be split into a 2 plasmid system for easier assembly.

Figure 1: Standard Blue LDR construct.

Figure 2: Blue LDR with inverter.
For the non-inverted construct the parts are as follows:
BBa_J23100 - constitutive promoter
We will use a σ70 constitutive promoter as this is the main E. coli sigma factor. Consequently, there should be RNA polymerase present to transcribe from this promoter at all stages during the bacterial growth cycle. Specifically, we have chosen BBa_J23100, an artificial promoter due to its widespread use, documentation and comparatively short sequence (35bp).
BBa_K592016 - FixJ & YF1 with RBSs
The YF1 and FixJ coding sequences are provided as a composite part together with standard RBSs in part BBa_K592016 which we have chosen for ease of assembly, in the event that we or future teams wish to use the BioBrick standard assembly to produce our part.
BBa_K592006 - FixK2
This is the wild-type promoter to which phosphorylated FixJ binds. It is reported that this promoter has very little leaky activity in the absence of FixJ.
BBa_K519010 - SmtA
This is the coding sequence for SmtA originally from Synechococcus sp, a cyanobacterial strain.
BBa_B1006 - Terminator
This is an artificial terminator part and was chosen because it has a high forward efficiency of 0.99.
pSB1C3 - Backbone
We are using the standard BioBrick backbone part pSB1C3 as this will make it easier to submit the part to the registry at a later date.
For the inverted part there are additional parts as follows:
BBa_C0051 - Lambda CI
This is the repressor protein from Lambda phage it represses the promoter BBa_R0051.
BBa_B0010 and BBa_B0012 - Double stop terminators
These are the terminators used in the composite part BBa_S04617 which is replicated in our construct.
BBa_R0051 - Lambda CI controlled promoter
This is a promoter from Lambda phage that is repressed by lambda Cl (BBa_C0051).
Construction
Synthesis
This construct can be sourced from IDT using our free allowance.
BioBrick Assembly
There exist a number of intermediate assembly components in the parts distribution that can be used to assemble our part faster if we use BioBrick assembly. Notably, BBa_S04617 contains the inverter,BBa_K592016 contains the FixJ and YF1. The two devices can be constructed separately as follows.
Constitutive Production Device
-
Cut the terminator BBa_B1006 with E & X.
-
Cut BBa_K592016 with E & S.
-
Mix & Ligate to form intermediate YF1FixJ+Terminator.
-
Cut the intermediate part with E & X and the constitutive promoter BBa_J23100 with E & S.
-
Mix and Ligate to form the constitutive production device.
SmtA Expression Device (inverted)
-
Cut BBa_K519010 with E & X.
-
Cut BBa_S04617 with E & S.
-
Mix and ligate to produce intermediate part: inverted smtA production.
-
Cut terminator with E & X.
-
Cut SmtA production intermediate with E & S.
-
Mix and ligate to produce SmtA expression device.
To produce the non-inverted device replace BBa_S04617 with the SmtA coding sequence and use an additional step to join this to an RBS, we suggest the standard RBS BBa_B0034 as this has good efficiency.
| Part No. | Name | Purpose |
|---|---|---|
| BBa_R0080 | L-Arabinose Promoter | This part is an L-Arabinose inducible promoter with very low level expression in the absence of L-Arabinose and AraC. Be aware, if the E. coli strain used constitutively expresses AraC then this promoter will ‘leak’. Check the strain list for information. |
| BBa_R0040 | TetR | This is the coding sequence for TetR which represses BBa_R0040. |
| BBa_R0040 | TetR Repressible Promoter | This is a constitutively on promoter which can be repressed by TetR. Be aware, if the strain used expresses TetR constitutively then this promoter will be repressed. Check the strain list for information. |
| BBa_C0052 | 434 Repressor | Represses the output promoter. |
| BBa_C0051 | Lambda Repressor | Induces the output promoter. |
| BBa_I12006 | Modified Promoter Part | This is a modified promoter part, originally the lambda Prm promoter. The modification allows it to be activated by lambda repressor and repressed by 434 repressor. |
| BBa_I746916 | Superfolder GFP | This is the coding sequence for super folder GFP. We have chosen to use this as our reporter because it can easily be quantified using a plate by taking the OD600 measurement. This is harder to quantify with more visible reporters like amilCP. |
| BBa_B1006 | Standard Terminator | We chose to use this promoter from the registry as it has a high forward efficiency. |
| BBa_B0034 | RBS | We chose to use this RBS from the registry as it is efficient and widely used in iGEM projects. |

