| Line 118: | Line 118: | ||
<h4>Results</h4> | <h4>Results</h4> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 12:01, 8 October 2016
Proof of Concept
Electrically Induced 'Light Bulb'
We plan to clone Escherichia coli so that it fluoresces when a current of around 400mA is passed through the broth that the E. coli are growing in.
Previous attempts at this experiment have used GFP to fluoresce with the current. In terms of this experiment we shall use GFP to ensure the design construct works. We have incorporated restriction sites within the construct so that we are able to then switch the GFP with other constructs, such as luciferase to emit light like an actual lightbulb.
In order for the GFP to be activated by a current, a heat-shock induced promoter must be present. Previous experiments have used sigma 32 as a heat shock promoter which seems to have the desired effect, although we shall test this in the lab. We have also inserted two restriction sites to allow us to either cut out the sigma 32 promoter, or add an extra promoter. This will allow us to test the effect that the sigma 32 has on the GFP production and whether it is induced by heat-shock or not.
The design has a natural ribosome binding site, which we will be adding to the registry (BBa_K1895001). This is to ensure that the ribosome does bind to the DNA and synthesise the protein correctly, however we will make variants of this DNA with two different medium bicistronic rbs. The medium bicistronic rbs will avoid the problem of placing too high a translational burden on the cell. We will then test all three variants to determine which is the best rbs to use in the final design.
Results


Arabinose Controlled 'Variable Resistor'
We plan to engineer Escherichia coli to behave like a variable resistor. We aim to do this by using E. coli to vary the amount of free ions in an electrolyte. Ion uptake will be controlled by the expression of smtA. SmtA is a metallothionein that can bind to heavy metal ions like cadmium (II), Zinc (II) and Copper (II).
SmtA has been used in a number of iGEM projects and is in the registry (BBa_K519010). It has previously been used in experiments for Cadmium (II) uptake, see Tokyo-NokoGen 2011. We will be examining firstly, the impact of smtA of Zinc (II) concertation rather than Cadmium (II) and then the impact that this has on the resistivity of the Zinc (II) containing media. In this instance we will be using Zinc sulfate (ZnSO4) in solution where it disassociates into Zn2+ and SO42- ions. Various concentrations of Zinc sulfate have known electrical conductivity . When smtA is expressed it will render the Zn2+ unavailable and thereby reduce the conductivity of the solution.
We will be placing smtA under the control of an AraC regulated promoter allowing the expression of smtA to be controlled by the addition or removal of arabinose.
Results
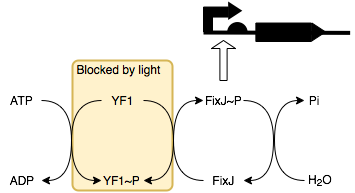 |
We will be placing smtA under the control of a FixJ-P (phosphorylated FixJ)
promoter. This allows it to be regulated by blue light through a series of
reactions with its response regulator protein YF1 (below).
In the absence of light, YF1 undergoes autophosphorylation to produce YF1-P which can then phosphorylate FixJ. This in turn activates the transcription of the downstream protein, in this case it is SmtA. Thus, in the presence of light SmtA is not produced and so conductivity does not change, whilst in the absence of light SmtA is produced resulting in a decrease in resistance.
Clearly, this behaviour is the inverse of an electrical light dependent resistor where resistance increases with light intensity. To mimic this behaviour using biological circuits we would place an inverter before the FixK2 promoter (which is activated by FixJ-P). The inverter is constructed by placing the desired output, here SmtA, under the control of a lambda cl regulated promoter (BBa_R0051). As lambda cl represses the promoter having this produced under control of FixK2 promoter inverts the system so that SmtA is produced in the presence of light rather than the absence thereof. BBa_K592020 is an example of a part that uses this technique.

