Project Overview
In 2010 it was estimated that 6.5 million people in the United States alone suffered from chronic wounds, accruing an annual cost of approximately $2.5 billion. Furthermore, experts predict that the burden of chronic wounds will increase rapidly in the near future due to increasing medical costs, an aging population, and the emergence of antibiotic resistant bacteria. Chronic wounds are characterized by their inability to progress through an orderly set of stages within a time period of about three months. Wound healing progresses through four successive stages known as hemostasis, inflammation, proliferation and remodeling.



Our Goals
We will use synthetic biology principles to help treat chronic wounds by targeting the overproduction of wound site protease.
- For Aim 1 we will genetically engineer E. coli to produce a protease inhibitor and platelet derived growth factor.
- For Aim 2 we will purify the protease inhibitor and platelet derived growth factor in a bioreactor.
- For Aim 3 we will design a collagen bandage that mimics the human extracellular matrix, and infuse it with purified protease inhibitor and platelet derived growth factor.
Aim 1
We were able to design constructs for two therapeutic proteins, aprotinin and platelet derived growth factor (PDGF). Our goal was to use these therapeutic proteins to decrease wound healing times. Aprotinin, being a matrix-metalloprotease (MMP) inhibitor, will inhibit wound-site proteases, preventing these proteases from breaking down the healing extra-cellular matrix (ECM). PDGF will stimulate cellular regeneration in chronic wounds, helping to speed up and fortify the wound healing process.




Aim 2
We designed, created, and tested a bioreactor to grow our bacteria in conditions so as to maximize protein production. With this optimal environment, we hoped to create a sustainable, cost-effective protocol to mass-produce our therapeutic agents.
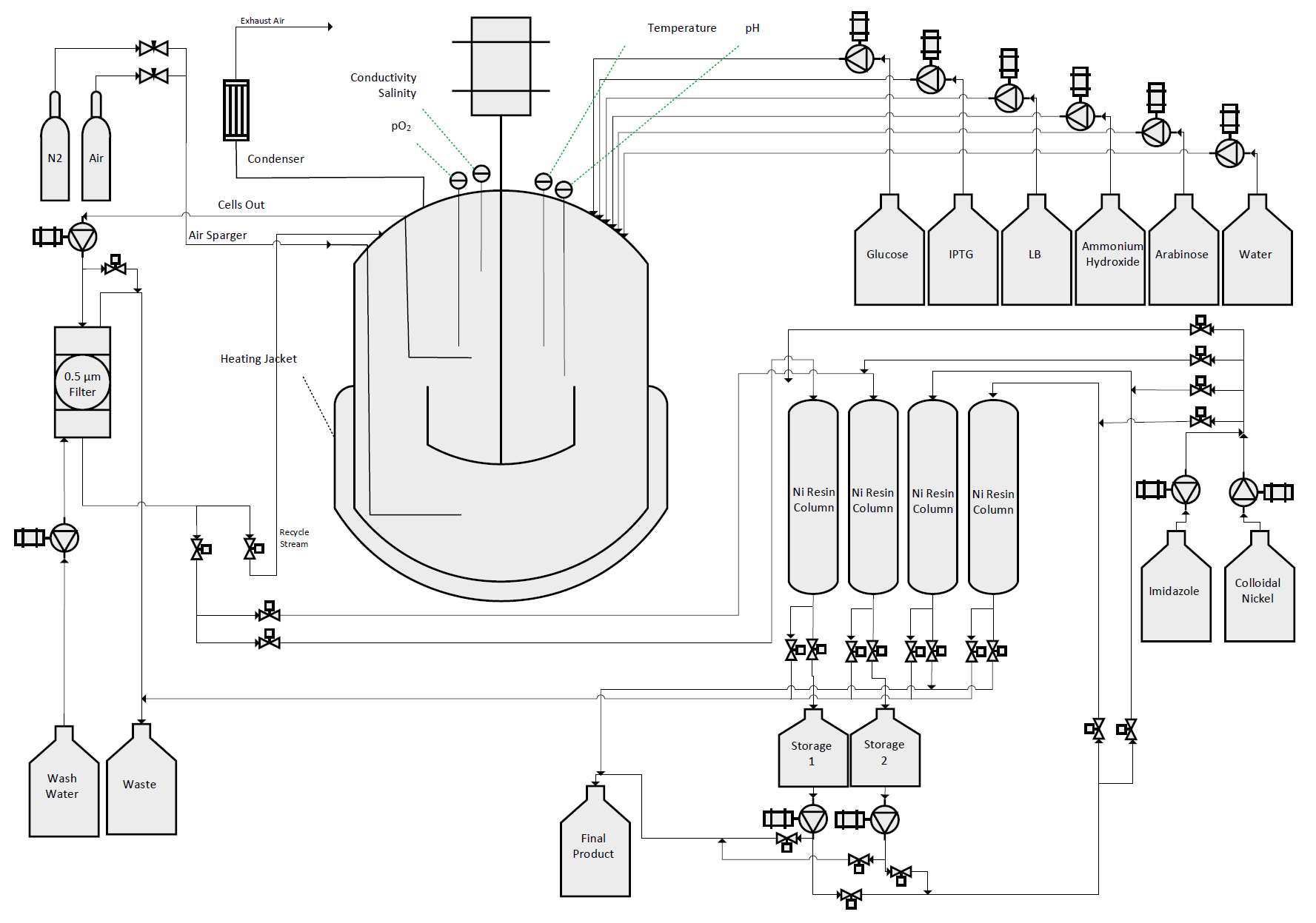



Aim 3
We made a control collagen matrix to compare with a collagen matrix that will be infused with our purified proteins, aprotinin and PDGF, which would imitate the human extracellular matrix. This would give the wound bed all the resources it would need for a speedy recovery.


Conclusion
Coupling a bioreactor with our Kil protein secretion method, we will be able to purify large quantities of DsbA tagged therapeutic protein from the supernatant with a continuous flow through process. Western blot analysis helped us to confirm the identity of our purified PDGF-B protein, and the bioreactor allowed us to produce large amounts of our secretion proteins.


