| (30 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lubbock_TTU/main}} | {{Lubbock_TTU/main}} | ||
| − | + | ||
<html> | <html> | ||
<style> | <style> | ||
| − | + | /* unvisited link */ | |
| − | + | a:link { | |
| − | + | color: #e00; | |
| − | + | } | |
| + | |||
| + | /* visited link */ | ||
| + | a:visited { | ||
| + | color: #e00; | ||
| + | } | ||
| + | |||
| + | /* mouse over link */ | ||
| + | a:hover { | ||
| + | color: #f66; | ||
| + | } | ||
</style> | </style> | ||
| Line 15: | Line 25: | ||
<div class="container-fluid"> | <div class="container-fluid"> | ||
| − | <!-- Section | + | <!-- Section 1 --> |
<div class="row" id="projintro" style="padding-top:0px;"> | <div class="row" id="projintro" style="padding-top:0px;"> | ||
<div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | ||
<h3>Project Overview</h3></br> | <h3>Project Overview</h3></br> | ||
| − | In 2010 | + | In 2010 it was estimated that 6.5 million people in the United States alone suffered from chronic wounds, accruing an annual cost of approximately $2.5 billion. Furthermore, experts predict that the burden of chronic wounds will increase rapidly in the near future due to increasing medical costs, an aging population, and the emergence of antibiotic resistant bacteria. Chronic wounds are characterized by their inability to progress through an orderly set of stages within a time period of about three months. Wound healing progresses through four successive stages known as hemostasis, inflammation, proliferation and remodeling. |
| − | + | ||
| − | + | ||
</br></br><center> | </br></br><center> | ||
<img class="fancybox" src="https://static.igem.org/mediawiki/2016/9/95/T--Lubbock_TTU--swh_1.jpg" style="width:24%" title="Image Source: Student Nurse Adventures"></img> | <img class="fancybox" src="https://static.igem.org/mediawiki/2016/9/95/T--Lubbock_TTU--swh_1.jpg" style="width:24%" title="Image Source: Student Nurse Adventures"></img> | ||
| Line 28: | Line 36: | ||
<img class="fancybox" src="https://static.igem.org/mediawiki/2016/7/70/T--Lubbock_TTU--swh_4.jpg" style="width:24%" title="Image Source: Student Nurse Adventures"></img> | <img class="fancybox" src="https://static.igem.org/mediawiki/2016/7/70/T--Lubbock_TTU--swh_4.jpg" style="width:24%" title="Image Source: Student Nurse Adventures"></img> | ||
</br></center></br> | </br></center></br> | ||
| − | + | The etiology of chronic wounds is very diverse, but patients frequently suffer from persisting chronic wounds arrested in the inflammation phase due to overproduction of wound site proteases. In turn, proteases inhibit the proliferation phase by degrading growth factors meant to induce tissue growth. They also inhibit tissue remodeling by degrading the collagen scaffold, which new cells migrate into. Thus, proteases decrease wound healing rates by degrading host growth factors and the extracellular matrix of the wound site. | |
| + | </br></br> | ||
| + | Our team is using a bioreactor and synthetic biology principles to purify and infuse a synthetic collagen scaffold with platelet derived growth factor (PDGF) and Aprotinin to induce the healing process of chronic wounds. Synthetic collagen scaffolds are currently being used as a replacement for skin grafts in the treatment of burn victims. They have been shown to increase wound healing by attracting tissue cells, such as keratinocytes and aiding in angiogenesis and re-epithelialization. | ||
| + | </br></br> | ||
| + | In our collagen scaffold, the aprotinin serves to prevent degradation from wound site proteases. Furthermore, recent studies have shown that aprotinin can increase angiogenesis, ultimately improving synthetic scaffold integration efficiency. PDGF has been well studied in the past and is the first growth factor to be approved by the FDA for human treatment. Currently ointments infused with PDGF, such as REGRANEX are being used to treat chronic wounds. We envision that our technology will help to introduce a novel synthetic-biology-based process for the development of therapeutic wound dressings. | ||
<a href="javascript:toggletext('mytext')">Learn more.</a> | <a href="javascript:toggletext('mytext')">Learn more.</a> | ||
| Line 39: | Line 51: | ||
</div></br> | </div></br> | ||
</div> <!-- End of projintro --> | </div> <!-- End of projintro --> | ||
| − | <!-- End of Section | + | <!-- End of Section 1 --> |
| − | <!-- Section | + | <!-- Section 2 --> |
| − | + | <a name="goals"> | |
| − | + | <div class="row" id="teamintro" style="Background:#f6f6f6;"> | |
| − | + | </br><h3 style="padding-top:0px;">Our Goals</h3> | |
| − | + | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | |
| − | <div class="row" id="teamintro" style="Background:# | + | <font color="#5d5d5d"> |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
We will use synthetic biology principles to help treat chronic wounds by targeting the overproduction of wound site protease. | We will use synthetic biology principles to help treat chronic wounds by targeting the overproduction of wound site protease. | ||
| Line 58: | Line 66: | ||
<li>For Aim 3 we will design a collagen bandage that mimics the human extracellular matrix, and infuse it with purified protease inhibitor and platelet derived growth factor.</li></ul></br> | <li>For Aim 3 we will design a collagen bandage that mimics the human extracellular matrix, and infuse it with purified protease inhibitor and platelet derived growth factor.</li></ul></br> | ||
| − | Our approach is two-fold. By infusing the collagen extracellular matrix with platelet derived growth factor and protease inhibitor, chronic wounds should be able to progress past the inflammation phase and begin healing once again. | + | Our approach is two-fold. By infusing the collagen extracellular matrix with platelet derived growth factor and protease inhibitor, chronic wounds should be able to progress past the inflammation phase and begin healing once again.</br></br> |
| − | <div id="readmore"><a href="">What We Accomplished →</a></br></br></br></div> | + | <div id="readmore"><a href="https://2016.igem.org/Team:Lubbock_TTU/Results">What We Accomplished →</a></br></div> |
| − | + | </font> | |
| − | + | </div> <!-- End of col-md-14 --> | |
| − | + | <div class="col-md-2"></div> | |
| − | + | </div> <!-- End of teamintro --> | |
| − | + | </div> <!-- End of Container-Fluid --> | |
| − | + | <!-- End of Section 2 --> | |
| − | + | ||
| − | + | <!-- Section 3 --> | |
| − | + | <div class="row" id="teamintro" style="Background:#eee;"> | |
| − | <!-- End of Section | + | </br></br><h3 style="padding-top:0px;">Aim 1</h3> |
| + | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | ||
| + | We were able to design constructs for two therapeutic proteins, aprotinin and platelet derived growth factor (PDGF). Our goal was to use these therapeutic proteins to decrease wound healing times. Aprotinin, being a matrix-metalloprotease (MMP) inhibitor, will inhibit wound-site proteases, preventing these proteases from breaking down the healing extra-cellular matrix (ECM). PDGF will stimulate cellular regeneration in chronic wounds, helping to speed up and fortify the wound healing process. | ||
| + | </br></br> | ||
| + | <center><img src="https://static.igem.org/mediawiki/2016/a/a4/T--Lubbock_TTU--dc2.png" width="350"></img> | ||
| + |   | ||
| + | <img src="https://static.igem.org/mediawiki/2016/0/09/T--Lubbock_TTU--dc4.png" width="350"></img></center> | ||
| + | </br><div id="readmore"><a href="https://2016.igem.org/Team:Lubbock_TTU/Experiments">Experimental Data →</a></br></div> | ||
| + | </div> <!-- End of col-md-14 --> | ||
| + | </br></br><div class="col-md-2"></div> | ||
| + | </div> <!-- End of teamintro --> | ||
| + | </div> <!-- End of Container-Fluid --> | ||
| + | <!-- End of Section 3 --> | ||
| + | |||
| + | <!-- Section 4 --> | ||
| + | <div class="row" id="teamintro" style="Background:#e6e6e6;"> | ||
| + | </br></br><h3 style="padding-top:0px;">Aim 2</h3> | ||
| + | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | ||
| + | We designed, created, and tested a bioreactor to grow our bacteria in conditions so as to maximize protein production. With this optimal environment, we hoped to create a sustainable, cost-effective protocol to mass-produce our therapeutic agents. | ||
| + | </br></br> | ||
| + | <center><img src="https://static.igem.org/mediawiki/2016/e/e7/T--Lubbock_TTU--dc1.png" width="350"></img> | ||
| + |   | ||
| + | <img src="https://static.igem.org/mediawiki/2016/7/71/T--Lubbock_TTU--dc5.png" width="350"></img></center> | ||
| + | </br><div id="readmore"><a href="https://2016.igem.org/Team:Lubbock_TTU/Experiments">Experimental Data →</a></br></div> | ||
| + | </div> <!-- End of col-md-14 --> | ||
| + | </br></br><div class="col-md-2"></div> | ||
| + | </div> <!-- End of teamintro --> | ||
| + | </div> <!-- End of Container-Fluid --> | ||
| + | <!-- End of Section 4 --> | ||
| + | |||
| + | <!-- Section 5 --> | ||
| + | <div class="row" id="teamintro" style="Background:#ddd;"> | ||
| + | </br></br><h3 style="padding-top:0px;">Aim 3</h3> | ||
| + | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | ||
| + | We made a control collagen matrix to compare with a collagen matrix that will be infused with our purified proteins, aprotinin and PDGF, which would imitate the human extracellular matrix. This would give the wound bed all the resources it would need for a speedy recovery. | ||
| + | </br></br> | ||
| + | <center><img src="https://static.igem.org/mediawiki/2016/8/85/T--Lubbock_TTU--dc3.png" width="350"></img></center> | ||
| + | </br><div id="readmore"><a href="https://2016.igem.org/Team:Lubbock_TTU/Experiments">Experimental Data →</a></br></div> | ||
| + | </div> <!-- End of col-md-14 --> | ||
| + | </br></br><div class="col-md-2"></div> | ||
| + | </div> <!-- End of teamintro --> | ||
| + | </div> <!-- End of Container-Fluid --> | ||
| + | <!-- End of Section 5 --> | ||
| + | |||
| + | <!-- Section 6 --> | ||
| + | <div class="row" id="teamintro" style="Background:#d5d5d5;"> | ||
| + | </br></br><h3 style="padding-top:0px;">Conclusion</h3> | ||
| + | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | ||
| + | Coupling a bioreactor with our Kil protein secretion method, we will be able to purify large quantities of DsbA tagged therapeutic protein from the supernatant with a continuous flow through process. Western blot analysis helped us to confirm the identity of our purified PDGF-B protein, and the bioreactor allowed us to produce large amounts of our secretion proteins. | ||
| + | </div> <!-- End of col-md-14 --> | ||
| + | </br></br><div class="col-md-2"></div> | ||
| + | </div> <!-- End of teamintro --> | ||
| + | </div> <!-- End of Container-Fluid --> | ||
| + | <!-- End of Section 6 --> | ||
| + | |||
| + | <!-- Section 7 --> | ||
| + | <div class="row" id="teamintro" style="Background:#ccc;"> | ||
| + | </br></br><h3 style="padding-top:0px;">Gold Medal Part Improvement</h3> | ||
| + | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | ||
| + | A big part of the 2016 TTU iGEM teams project relied upon being able to produce Aprotinin (BBa_K1456006), a protease inhibitor for infusion into a collagen scaffold. To increase our chances we decided to improve upon this part which was created in 2014 by the ATOMS-Turkiye iGEM team. | ||
| + | |||
| + | </br></br> | ||
| + | The first thing we did to improve this part was to remove two illegal sites to make it compatible with all iGEM RFC standards. Secondly, we found out that Aprotinin contains three disulphide bonds, which make correct folding in the cytoplasm difficult. To overcome this problem we decided to add a periplasmic localization peptide to K1456006, which allows for cotranslational translocation into the periplasm, where disulphide bonds are more easily formed. | ||
| + | |||
| + | </br></br> | ||
| + | <center><img src="https://static.igem.org/mediawiki/2016/7/7f/T--Lubbock_TTU--ptimpr.png" width="450"></img></center> | ||
| + | |||
| + | </div> <!-- End of col-md-14 --> | ||
| + | </br></br><div class="col-md-2"></div> | ||
| + | </div> <!-- End of teamintro --> | ||
| + | </div> <!-- End of Container-Fluid --> | ||
| + | <!-- End of Section 7 --> | ||
| − | <!-- Section | + | <!-- Section 8 --> |
<!-- Project Start --> | <!-- Project Start --> | ||
| − | <div class="row" id="teamintro" style="Background:# | + | <div class="row" id="teamintro" style="Background:#c4c4c4;"> |
</br><h3 style="padding-top:0px;">References</h3> | </br><h3 style="padding-top:0px;">References</h3> | ||
<div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | <div class="col-md-14 content" style="max-width:1000px;padding:10px 50px;"> | ||
| − | + | ||
<ul> | <ul> | ||
| − | <li> | + | <li>Bowler, P. G., B. I. Duerden, and David G. Armstrong. "Wound microbiology and associated approaches to wound management." Clinical microbiology reviews 14, no. 2 (2001): 244-269.</il> |
| − | <li> | + | |
| − | <li> | + | <li>Chen, Xiaoying, Jennica L. Zaro, and Wei-Chiang Shen. "Fusion protein linkers: property, design and functionality." Advanced drug delivery reviews65, no. 10 (2013): 1357-1369.</il> |
| + | |||
| + | <li>Cutting, Keith F., and Richard White. "Defined and refined: criteria for identifying wound infection revisited." Br J Community Nurs 9, no. 3 (2004): S6-15.</il> | ||
| + | |||
| + | <li>Fivenson, David P., Duyen T. Faria, Brian J. Nickoloff, Peter J. Poverini, Steven Kunkel, Marie Burdick, and Robert M. Strieter. "Chemokine and inflammatory cytokine changes during chronic wound healing." Wound Repair and Regeneration 5, no. 4 (1997): 310-322.</il> | ||
| + | |||
| + | <li>Goldman, Robert. "Growth factors and chronic wound healing: past, present, and future." Advances in skin & wound care 17, no. 1 (2004): 24-35.</il> | ||
| + | |||
| + | <li>Harley, Brendan A., Janet H. Leung, Emilio CCM Silva, and Lorna J. Gibson. "Mechanical characterization of collagen–glycosaminoglycan scaffolds." Acta biomaterialia 3, no. 4 (2007): 463-474.</il> | ||
| + | |||
| + | <li>Harley, Brendan AC, and Lorna J. Gibson. "In vivo and in vitro applications of collagen-GAG scaffolds." Chemical Engineering Journal 137, no. 1 (2008): 102-121.</il> | ||
| + | |||
| + | <li>Hortensius, Rebecca A., and Brendan AC Harley. "Naturally derived biomaterials for addressing inflammation in tissue regeneration."Experimental Biology and Medicine (2016): 1535370216648022.</il> | ||
| + | |||
| + | <li>Johnson, A. Wagoner, and Brendan Harley, eds. Mechanobiology of cell-cell and cell-matrix interactions. Springer Science & Business Media, 2011.</il> | ||
| + | |||
| + | <li>O'Brien, Fergal J., Brendan A. Harley, Mary A. Waller, Ioannis V. Yannas, Lorna J. Gibson, and Patrick J. Prendergast. "The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering."Technology and Health Care 15, no. 1 (2007): 3-17.</il> | ||
| + | |||
| + | <li>O’Brien, Fergal J., Brendan A. Harley, Ioannis V. Yannas, and Lorna Gibson. "Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds." Biomaterials 25, no. 6 (2004): 1077-1086.</il> | ||
| + | |||
| + | <li>Yager, Dorne R., Stephen M. Chen, Susan I. Ward, Oluyinka O. Olutoye, Robert F. Diegelmann, and I. Kelman Cohen. "Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors." Wound Repair and Regeneration 5, no. 1 (1997): 23-32.</il> | ||
| + | |||
| + | <li>Yannas, I. V., D. S. Tzeranis, B. A. Harley, and P. T. C. So. "Biologically active collagen-based scaffolds: advances in processing and characterization."Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 368, no. 1917 (2010): 2123-2139.</il> | ||
</ul> | </ul> | ||
| + | |||
</div> <!-- End of col-md-14 --> | </div> <!-- End of col-md-14 --> | ||
| − | <div class="col-md-2"></div> | + | </br></br><div class="col-md-2"></div> |
</div> <!-- End of teamintro --> | </div> <!-- End of teamintro --> | ||
| − | |||
</div> <!-- End of Container-Fluid --> | </div> <!-- End of Container-Fluid --> | ||
| + | <!-- End of Section 8 --> | ||
</body> | </body> | ||
Latest revision as of 03:28, 20 October 2016
Project Overview
In 2010 it was estimated that 6.5 million people in the United States alone suffered from chronic wounds, accruing an annual cost of approximately $2.5 billion. Furthermore, experts predict that the burden of chronic wounds will increase rapidly in the near future due to increasing medical costs, an aging population, and the emergence of antibiotic resistant bacteria. Chronic wounds are characterized by their inability to progress through an orderly set of stages within a time period of about three months. Wound healing progresses through four successive stages known as hemostasis, inflammation, proliferation and remodeling.



Our Goals
We will use synthetic biology principles to help treat chronic wounds by targeting the overproduction of wound site protease.
- For Aim 1 we will genetically engineer E. coli to produce a protease inhibitor and platelet derived growth factor.
- For Aim 2 we will purify the protease inhibitor and platelet derived growth factor in a bioreactor.
- For Aim 3 we will design a collagen bandage that mimics the human extracellular matrix, and infuse it with purified protease inhibitor and platelet derived growth factor.
Aim 1
We were able to design constructs for two therapeutic proteins, aprotinin and platelet derived growth factor (PDGF). Our goal was to use these therapeutic proteins to decrease wound healing times. Aprotinin, being a matrix-metalloprotease (MMP) inhibitor, will inhibit wound-site proteases, preventing these proteases from breaking down the healing extra-cellular matrix (ECM). PDGF will stimulate cellular regeneration in chronic wounds, helping to speed up and fortify the wound healing process.




Aim 2
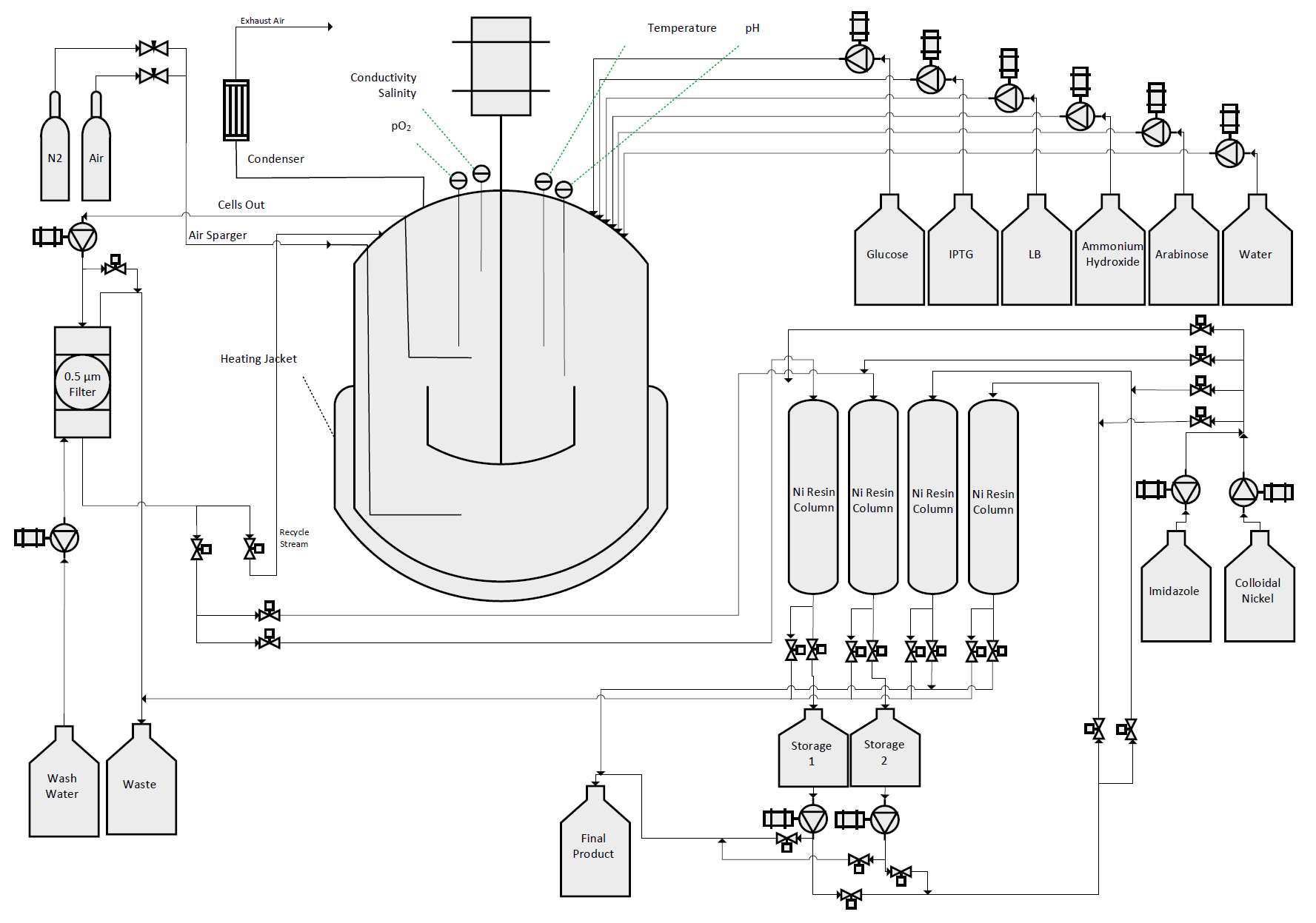
We designed, created, and tested a bioreactor to grow our bacteria in conditions so as to maximize protein production. With this optimal environment, we hoped to create a sustainable, cost-effective protocol to mass-produce our therapeutic agents.



Aim 3
We made a control collagen matrix to compare with a collagen matrix that will be infused with our purified proteins, aprotinin and PDGF, which would imitate the human extracellular matrix. This would give the wound bed all the resources it would need for a speedy recovery.


Conclusion
Coupling a bioreactor with our Kil protein secretion method, we will be able to purify large quantities of DsbA tagged therapeutic protein from the supernatant with a continuous flow through process. Western blot analysis helped us to confirm the identity of our purified PDGF-B protein, and the bioreactor allowed us to produce large amounts of our secretion proteins.


