(Okay 1) |
|||
| Line 119: | Line 119: | ||
E.coli is intended to be the “Plan B” host organism, in case protein expression in B.subtilis cannot be achieved. | E.coli is intended to be the “Plan B” host organism, in case protein expression in B.subtilis cannot be achieved. | ||
| − | An inductive promoter – “pLac” – is coupled with a sequence coding for ribosome binding site (RBS | + | An inductive promoter – “pLac” – is coupled with a sequence coding for ribosome binding site (RBS). This whole sequence, so called pLac-RBS (Part: BBa_K2029014), was synthesised at Integrated DNA Technologies (IDT), thanks to their sponsorship. |
| − | {{UBonn_HBRS/image|url=//2016.igem.org/wiki/images/0/01/T--UBonn_HBRS--Cloning_general_strategy_e_coli.png|alt=Strategy E. coli|class= | + | {{UBonn_HBRS/image|url=//2016.igem.org/wiki/images/0/01/T--UBonn_HBRS--Cloning_general_strategy_e_coli.png|alt=Strategy E. coli|class=50 borderless|caption-class=with-caption|caption=<strong>Figure 2. Cloning strategy in E. coli</strong>}} |
| + | |||
| + | <html><div class="clearfix"></div></html> | ||
| Line 129: | Line 131: | ||
| − | {{UBonn_HBRS/image|url=//2016.igem.org/wiki/images/6/61/T--UBonn_HBRS--Cloning_backbone_gel.png|alt=Backbone on gel|class= | + | {{UBonn_HBRS/image|url=//2016.igem.org/wiki/images/6/61/T--UBonn_HBRS--Cloning_backbone_gel.png|alt=Backbone on gel|class=50 borderless|caption-class=with-caption|caption=<strong>Figure 3. Gel electrophoresis of pSBbs1AK1 with a mazF insert, showing linearisation of the plasmid when cut with the BioBrick sites E, P, X, S, as well as K and H, and two fragments in double digestions.</strong>}} |
| + | |||
| + | <html><div class="clearfix"></div></html> | ||
| Line 203: | Line 207: | ||
'''Table 3. Data from an experiment showing growth of E.coli with P34-nprb and P34-SacB in pSB4C5 (“C5”), as opposed to in pSB1C3 (“C3”)''' | '''Table 3. Data from an experiment showing growth of E.coli with P34-nprb and P34-SacB in pSB4C5 (“C5”), as opposed to in pSB1C3 (“C3”)''' | ||
<html></div></html> | <html></div></html> | ||
| + | |||
| + | |||
| + | With the ''E. coli'' track, ligation of the pLac-RBS construct with GOIs did not yield any colony until the very last week of work in the laboratory, so even though colonies have appeared, it has not been ascertained whether they hold the desired plasmids. | ||
| + | |||
| + | Due to the lack of cloning success so far, no enzyme has been produced by bacterial cells, and the cloning sub-area has not been able to provide input to other aspects of the project, namely protein expression and purification in B.subtilis, deinking and enzyme assay. | ||
{{UBonn_HBRS/footer}} | {{UBonn_HBRS/footer}} | ||
Revision as of 01:05, 20 October 2016
Cloning
Contents
General Abstract
The cloning aspect of this project is responsible for programming bacterial cells to secrete enzymes which can decouple ink particles from paper fibers.
To this end, genes coding for enzymes were synthesised and ligated into a plasmid backbone, which is designed for use in both Escherichia coli and Bacillus subtilis.
As expression of enzymes is intended for both Bacillus subtilis and E.coli, there are two separate tracks for cloning. For B. subtilis, the genes were assembled into pSBbs1AK1 along with secretory tags, and for E.coli, an inductive promoter (pLac) is placed upstream of the genes.
Genes of Interest
Within the scope of this project, genes of interest (GOIs) are those which catalyse either cellulolysis of paper fiber, or hydrolysis of ink particles.
Synthesised at GenScript, each of these genes includes a sequence coding for His-tag, for ease of protein purification.
Cellulase group
Enzymes which break down cellulose molecules into monosaccharides, therefore partially disintegrate paper fibers and set ink particles free into the supernatant.
| Gene name | Enzyme coded | Part number | Note |
| XynA | Xylanase | BBa_K2029002 | http://www.microbialcellfactories.com/content/11/1/66 |
| CelA | Cellulase | BBa_K2029004 | https://www.ncbi.nlm.nih.gov/pubmed/22101447 |
| EngB | Endoglucanase | BBa_K2029005 | http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1785254/ |
| BsCel5c | Cellulase | BBa_K2029006 | http://www.ncbi.nlm.nih.gov/pubmed/21549854 |
Esterase-Laccase group
Enzymes which hydrolyse esters or oxidise phenols and aromatic compounds found in commercial inks. Their ability to attack ink particles is not well studied.
| Gene name | Enzyme coded | Part number | Note |
| EstC2 | Esterase | BBa_K2029007 | https://www.ncbi.nlm.nih.gov/pubmed/21619698 |
| LipA | Lipase | BBa_K2029008 | http://onlinelibrary.wiley.com/doi/10.1002/bit.10201/abstract |
| Bpul | Laccase | BBa_K2029009 | https://www.ncbi.nlm.nih.gov/nucleotide/388482909 |
General Strategy
The primary target chassis organism is B. subtilis, for which a secretory tag system was designed. At first E. coli was only intended to be used as the chassis for cloning, but as the project went on, E.coli was decided on as the backup system in case gene expression in B.subtilis fails.
In B. subtilis
As they express secretory proteins, GOIs require a signaling system in order to function in B.subtilis.
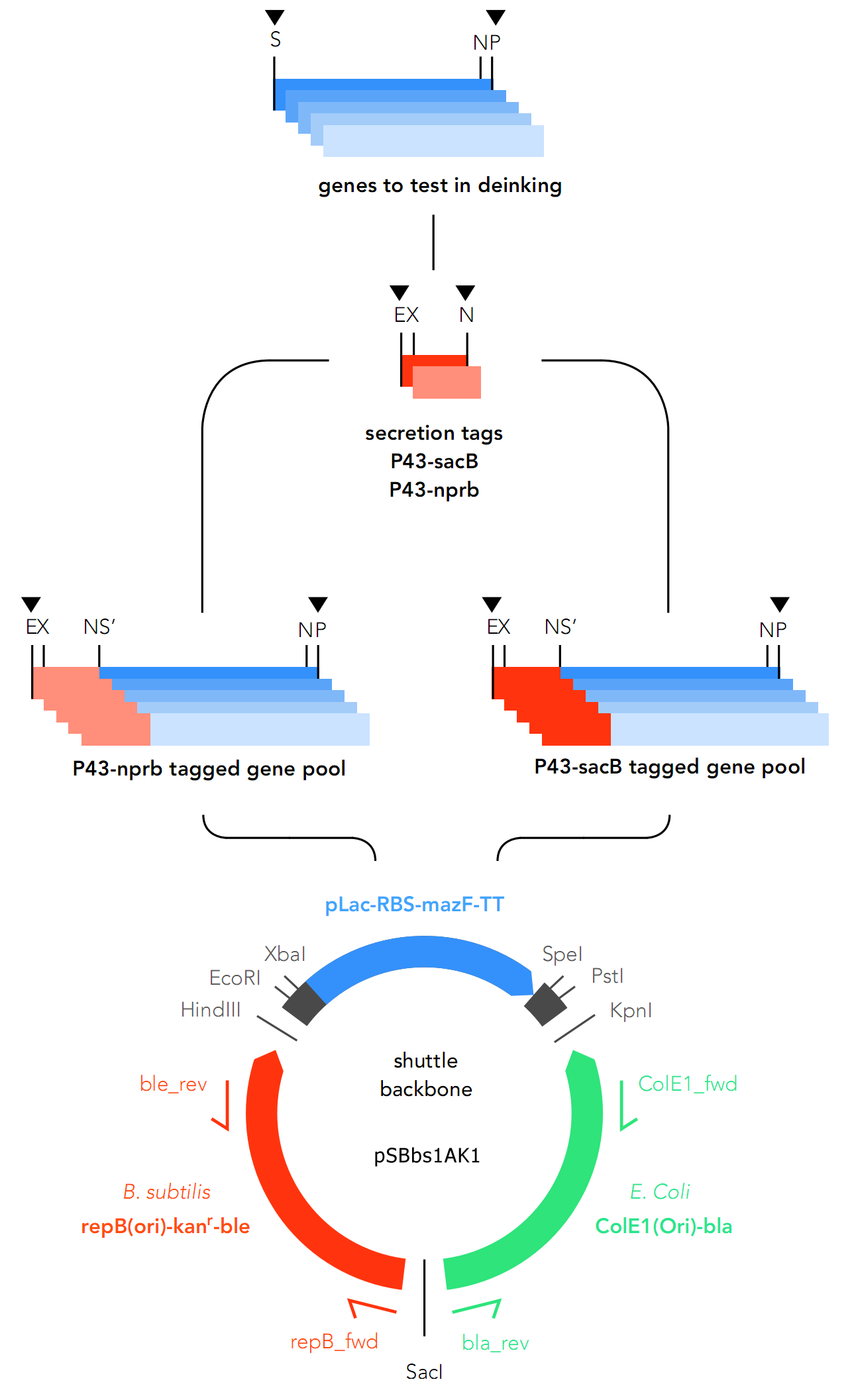
For this reason, two secretory tags are chosen to precede the genes: P34-sacB (Part: BBa_K2029001) and P34-nprb (BBa_K2029002). Both are constitutive in nature, which means they cannot be deactivated.
In E. coli
E.coli is intended to be the “Plan B” host organism, in case protein expression in B.subtilis cannot be achieved.
An inductive promoter – “pLac” – is coupled with a sequence coding for ribosome binding site (RBS). This whole sequence, so called pLac-RBS (Part: BBa_K2029014), was synthesised at Integrated DNA Technologies (IDT), thanks to their sponsorship.
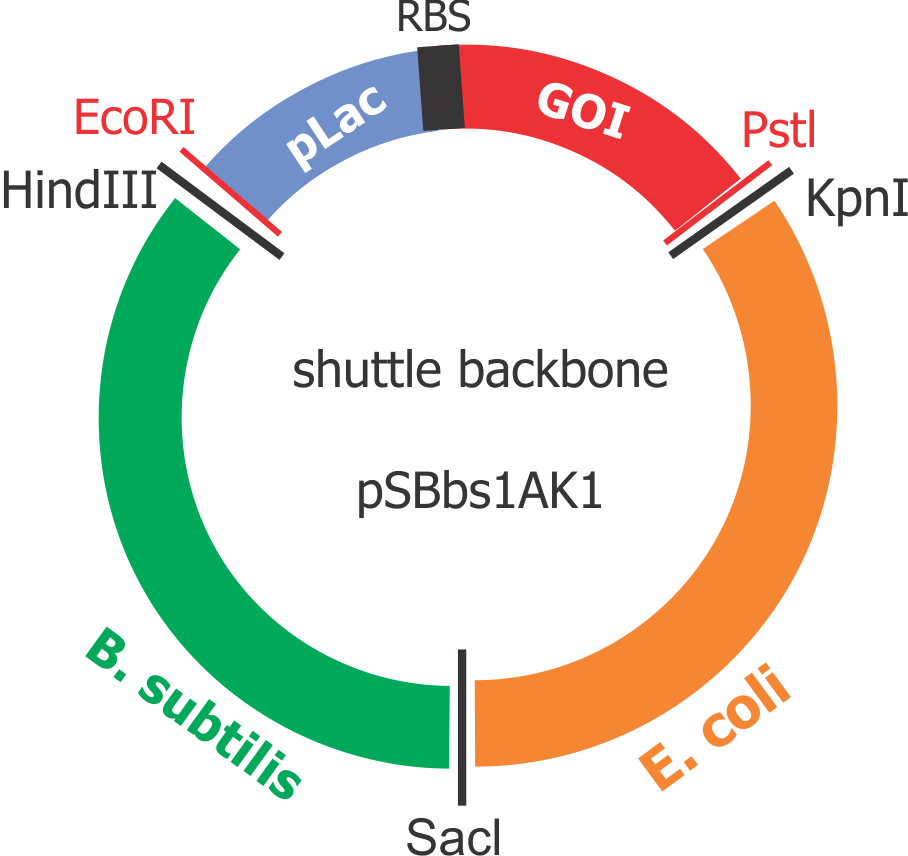
Composite “shuttle” plasmid backbone
As the GOIs are intended to be put into both chassis – E. coli and B. subtilis, a so-called “shuttle” backbone was developed to ensure compatibility with both species. This plasmid backbone - now given the nomenclature pSBbs1AK1 in the iGEM part registry – contains two different origins of replication, as well as confers resistance to two different antibiotics, one for each organism: against Kanamycin for B. subtilis and against Ampicillin for E. coli.
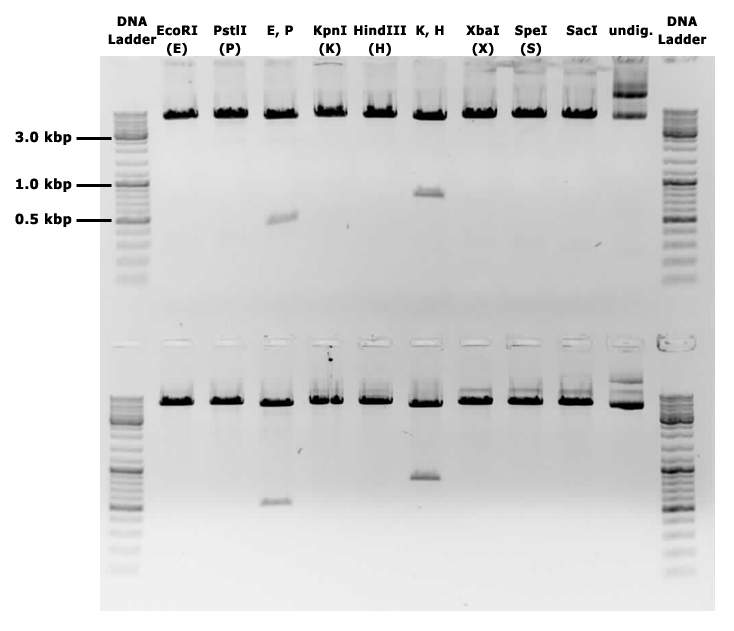
This backbone, however, has proven to be unsuitable for the gene assembly process due to its large size (appr. 5.5 kbp), hence all tag-gene constructs for B.subtilis and pLac-RBS-gene constructs for E.coli were assembled using existing iGEM backbones such as pSB1C3 and pSB4C5, and only transferred into pSBbs1AK1 in the final step.
Discussion
A major limitation of this cloning project is the lack of success, up until this point, in either aforementioned tracks for B. subtilis and E. coli.
With the B. subtilis track, while a total of 3 tag-gene constructs (Parts: BBa_K2029011, BBa_K2029012, BBa_K2029013) have been obtained, none of which has been successfully cloned into B. subtilis.
It is worth noting that all three constructs obtained contain the P34-nprb tag, and none with P34-SacB. Data showed there was little to no colony growth with pSB1C3 plasmids containing these tag-gene constructs. It is hypothesised that as these secretory tags are constitutive, protein expression overwhelms the E. coli cells used for assembly, and ultimately killed them. In a change of strategy, the tags were cloned into pSB4C5, a low-copy iGEM plasmid, which should reduce protein expression in theory. Results showed a noticeable improvement with P34-nprb, but no significant improvement in P34-SacB:
| Sample | Plate 1 | Plate 2 |
| SacB + C3 | 0 | 0 |
| SacB + C3 | 4 | 4 |
| nprb + C3 | 0 | 0 |
| nprb + C5 | 700 | 300 |
| Controls | ||
| SacB | 0 | |
| nprb | 0 | |
| C3 | 0 | |
| C5 | 0 | |
With the E. coli track, ligation of the pLac-RBS construct with GOIs did not yield any colony until the very last week of work in the laboratory, so even though colonies have appeared, it has not been ascertained whether they hold the desired plasmids.
Due to the lack of cloning success so far, no enzyme has been produced by bacterial cells, and the cloning sub-area has not been able to provide input to other aspects of the project, namely protein expression and purification in B.subtilis, deinking and enzyme assay.


