| Line 16: | Line 16: | ||
<p class = "h2WM">Project Results</p> | <p class = "h2WM">Project Results</p> | ||
<p><b>We divided our synthetic biology work into three sub-projects:</b> | <p><b>We divided our synthetic biology work into three sub-projects:</b> | ||
| − | <br><br>1. Development of a | + | <br><br>1. Development of a PSII-binding DNA aptamer (based on in silico prediction) |
| − | + | <br><br>2. Development of a graphene-binding DNA aptamer (using SELEX) | |
| − | <br><br>3. Characterisation of novel laccase enzymes that might be used to reduce oxygen and hydrogen back to water on the cathode electrode of our PBEC | + | <br><br>3. Characterisation of novel laccase enzymes that might be used to reduce oxygen and hydrogen back to water on the cathode electrode of our PBEC. |
</p> | </p> | ||
</div> | </div> | ||
<center> | <center> | ||
<img width="300" height="210" src="https://static.igem.org/mediawiki/2016/0/09/T--Pretoria_UP--DecoPlate.jpg"></center> | <img width="300" height="210" src="https://static.igem.org/mediawiki/2016/0/09/T--Pretoria_UP--DecoPlate.jpg"></center> | ||
| − | + | ||
| + | |||
<div class="section section-we-are-1"> | <div class="section section-we-are-1"> | ||
<div class="text-area"> | <div class="text-area"> | ||
| Line 29: | Line 30: | ||
<div class="row"> | <div class="row"> | ||
<div class="title"> | <div class="title"> | ||
| − | <p class = "h2WM"> | + | <p class = "h2WM">Development of a PSII-binding DNA aptamer</p> |
</div> | </div> | ||
<div class="col-sm-12"> | <div class="col-sm-12"> | ||
<p style="text-align:left;"> | <p style="text-align:left;"> | ||
| − | <b> | + | <b>Designing a DNA aptamer for Photosystem II (PSII)</b> |
<br>We chose PSII rather than Photosystem I (PSI) as our system of choice as it only requires water for energy generation whereas PSI requires alternative co-factors. We then analysed one dimer of the PSII using Pymol and started off with B-factor analysis. B-factor refers to the displacement of atoms from their mean position in a crystal structure (Trueblood <i>et al.</i>, 1996) . Using this analysis tool, we were able to determine which regions of the PSII complex are the most rigid and structurally stable and hence most suitable for aptamer-mediated targeting. | <br>We chose PSII rather than Photosystem I (PSI) as our system of choice as it only requires water for energy generation whereas PSI requires alternative co-factors. We then analysed one dimer of the PSII using Pymol and started off with B-factor analysis. B-factor refers to the displacement of atoms from their mean position in a crystal structure (Trueblood <i>et al.</i>, 1996) . Using this analysis tool, we were able to determine which regions of the PSII complex are the most rigid and structurally stable and hence most suitable for aptamer-mediated targeting. | ||
| Line 42: | Line 43: | ||
<br><br>The result of the B-factor analysis revealed two proteins in the complex that were structurally more stable than the rest, namely CP47 and CP43. We decided to target the CP43 and CP47 subunits of the light-harvesting complex (LHC) of PSII to which the one side of the aptamers can bind. This protein is suitable as it is exposed and on the stromal side of the thylakoid membranes (which is important because electrons can more easily be abstracted on the stromal side). These proteins form the core subunits of the PSII complex and we determined them to be the most stable. We used PDF files from a recent crystal structure of the PSII complex (Wei <i>et al.</i>, 2016) as the basis for aptamer design. iGEM team <a href="https://2015.igem.org/Team:Heidelberg">Heidelberg (2015)</a> used their designed MAWS (Making Aptamers Without SELEX) software to design an aptamer sequence which has specifically bind to CP43 and CP47 subunits of PSII. | <br><br>The result of the B-factor analysis revealed two proteins in the complex that were structurally more stable than the rest, namely CP47 and CP43. We decided to target the CP43 and CP47 subunits of the light-harvesting complex (LHC) of PSII to which the one side of the aptamers can bind. This protein is suitable as it is exposed and on the stromal side of the thylakoid membranes (which is important because electrons can more easily be abstracted on the stromal side). These proteins form the core subunits of the PSII complex and we determined them to be the most stable. We used PDF files from a recent crystal structure of the PSII complex (Wei <i>et al.</i>, 2016) as the basis for aptamer design. iGEM team <a href="https://2015.igem.org/Team:Heidelberg">Heidelberg (2015)</a> used their designed MAWS (Making Aptamers Without SELEX) software to design an aptamer sequence which has specifically bind to CP43 and CP47 subunits of PSII. | ||
| − | <br><br>We then removed all non-target proteins and cofactors from the PSII PDB file using Pymol, so that we were left with CP43 and CP47 subunits alone. This file was then sent to Heidelberg 2015 iGEM team who used it as a template for aptamer targeting using their MAWS software. At first the PSII protein complex was too large for the program to compute a prediction, so we reduced the size of the PDB file to target only the stromal side of CP47. This was used as input for which was then ran through the MAWS software to test for the best aptamer oligo sequence. | + | <br><br>We then removed all non-target proteins and cofactors from the PSII PDB file using Pymol, so that we were left with CP43 and CP47 subunits alone. This file was then sent to Heidelberg 2015 iGEM team who used it as a template for aptamer targeting using their MAWS software. At first the PSII protein complex was too large for the program to compute a prediction, so we reduced the size of the PDB file to target only the stromal side of CP47. This was used as input for which was then ran through the MAWS software to test for the best aptamer oligo sequence. The predicted aptamer candidate had the sequence 5' TACGTTAT 3'. |
| + | |||
</p> | </p> | ||
| + | <br><br><center><img width="500" height="400" src="https://static.igem.org/mediawiki/parts/c/c6/T-Pretoria_UP--PSII_core.png"></center> | ||
| + | <p style="font-size:14px !important;text-align: center">Figure depicting the core PSII components, with different colours representing distinct subunits of the complex. Pink, CP47; Cyan, CP43; green, D1; yellow, D2. Image drawn in Pymol. | ||
| + | </p> | ||
| + | |||
| + | <br><br><center><img width="500" height="400" src="https://static.igem.org/mediawiki/parts/e/e4/T--Pretoria_UP--aptamer_binding.png"></center> | ||
| + | <p style="font-size:14px !important;text-align: center">Figure depicting the predicted binding of the single stranded DNA aptamer candidate designed by the MAWS software (5' TACGTTAT 3'). The aptamer was predicted to bind to the stromal side of the CP47 subunit. Image drawn in Pymol. | ||
| + | </p> | ||
| − | < | + | <div class="col-sm-12"> |
| + | <p style="text-align:left;"> | ||
| + | <b>Isolation of thylakoids</b> | ||
| + | <br>Thylakoid extraction was carried out on store-bought spinach, using a protocol designed to extract and isolate intact chloroplasts from leaves, which we derived from Lang <i>et al.</i> (2011), Nagahashi <i>et al.</i> (1985), Rödiger <i>et al.</i> (2010) and Shiraya <i>et al.</i> (2014) as described below . The first protocol used involved grinding frozen tissue with a homogenizer in liquid nitrogen. A volume of 30ml of a “homogenization buffer” was added to 2 - 5 g of tissue, vortexed for 30 seconds and filtered through two layers of 100 µm nylon mesh, followed by two layers of 60 µm mesh. The filtrate was centrifuged at 200 x g for 4 minutes at 4°C, and the supernatant was then centrifuged for 10 minutes at 1500 x g at 4°C. The resulting pellet contained the chloroplasts. The pellet was gently resuspended in 3ml of “resuspension buffer”. Next the plastid suspension was slowly layered onto a discontinuous Percoll density gradient solution, containing equal volumes (9ml) of 85%, 40% and 10% Percoll solutions. The Percoll density gradient solution was then centrifuged at 5000 x g for 45 min at 4°C, after this centrifugation, intact chloroplasts were located on the interface of the 40/85 Percoll and the 10/40 interface contained broken chloroplasts and thylakoid membranes. Both fractions were gently transferred to separate falcon tubes, to which 3 volumes of “wash buffer” was added and then gently washed by centrifugation at 1500 x g for 10 min at 4°C (repeated once). The pellet was then resuspended in wash buffer and 50% glycerol solution for storage. | ||
| + | <br><br>This method extracted extremely pure, intact chloroplasts, but in small yields. Because we only required thylakoid membranes, not intact chloroplasts, separation using a Percoll gradient was not necessary. Furthermore, the protocol used small volumes of material and therefore resulted in a small amount of product. We therefore simplified and scaled up the protocol for crude thylakoid extractions: | ||
| − | <br><br> | + | <br><br>- A larger amount of material (5 - 10 g) was used |
| − | + | <br>- Separation via the percoll density gradient was omitted | |
| + | <br>- The first crude pellet containing the chloroplasts and thylakoids was resuspended in the “wash buffer” and then sonicated to disrupt the chloroplast membrane and therefore increase the amount of free thylakoids in the solution. | ||
| − | <br><br><b>Synthesis, cloning and expression of <i>Eucalyptus grandis</i> laccases</ | + | <br><br>The recipes of the buffers used may be seen in the tables below: |
| − | <br>We searched for <i>Eucalyptus grandis</i> homologs of LAC4 and LAC17, two <i>Arabidopsis thaliana</i> laccase proteins known to reduce phenolic compounds much like the laccases from Trametes versicolor traditionally used in PBECs (Berthet et al. 2011). | + | <br> |
| + | </p> | ||
| + | |||
| + | <p style="text-align:center;"> | ||
| + | <img style="max-width:40%" src="https://static.igem.org/mediawiki/2016/4/48/T--Pretoria_UP--Results_3.png"> | ||
| + | <img style="max-width:40%" src="https://static.igem.org/mediawiki/2016/e/e5/T--Pretoria_UP--Results_4.png"> | ||
| + | <img style="max-width:40%" src="https://static.igem.org/mediawiki/2016/7/75/T--Pretoria_UP--Results_5.png"> | ||
| + | <img style="max-width:40%" src="https://static.igem.org/mediawiki/2016/5/59/T--Pretoria_UP--Results_6.png"> | ||
| + | <br> | ||
| + | </p> | ||
| + | |||
| + | <p style="text-align:center;"> | ||
| + | <img height="500px" src="https://static.igem.org/mediawiki/2016/c/c5/T--Pretoria_UP--Results_2.png"> | ||
| + | <p style="font-size:14px !important;text-align: center">Thylakoid extractions from spinach leaves. (Left) Percoll gradient-mediated purification. The top green layer contains a mixture of thylakoid membranes and broken chloroplasts, while the bottom layer contains pure intact chloroplasts. (Right) The resulting product of the simplified extraction. | ||
| + | </p> | ||
| + | |||
| + | <br><br> | ||
| + | |||
| + | <div class="col-sm-12"> | ||
| + | <p style="text-align:left;"> | ||
| + | <b>Assessment of thylakoid binding by candidate aptamer</b> | ||
| + | <br>1. We synthesized the predicted PSII-binding aptamer (5' TACGTTAT 3') with an extra "foot" of random nucleic acids of 25-nt in length to give it a foothold to a nitrocellulose membrane. As a negative control, we synthesized a random oligo of the same length. (Reagents/materials used: | ||
| + | - Aptamers (N)25TACGTTAT: Stock (100uM) and working solution (10uM) | ||
| + | Random sequence as negative control | ||
| + | Nitrocellulose membrane | ||
| + | Thylakoids | ||
| + | Cherring testis DNA (1ul diluted in 20ml TBS) | ||
| + | TBS: | ||
| + | 5% (m/v) non-fat milk in TBS | ||
| + | |||
| + | Procedure: | ||
| + | <br>2. We made serial dilutions of the aptamers and negative control sequences from the 10 uM working solution: undiluted, 1/10, 1/100, 1/500, 1/1000. | ||
| + | <br>3. The nitrocellulose membrane was cut to the desired size, marked and spotted with 3 ul of aptamer solution. As a positive control, the thylakoid extraction was spotted directly onto the membrane. | ||
| + | <br>4. Non-specific sites were blocked by incubating it with the herring testis DNA for 30 min at 37°C followed by three wash steps with TBS (50 mM Tris-HCl pH 7.5, 150 mM NaCl). | ||
| + | <br>5. The blot was further blocked with 5% m/v nonfat milk solution followed by washing three times with TBS. | ||
| + | <br>6. The membrane was incubated in the thylakoid solution (diluted in TBS containing protease-inhibitor) on ice for 2 hours. It was then washed 3 times for 5 min with TBS at 4°C to remove any unbound thylakoids. | ||
| + | <br>7. The blots were imaged with a Carl Zeiss LSM 5 confocal microscope, excitation wavelength 395 nm, to observe chlorophyll autofluorescence of the bound thylakoids. | ||
| + | |||
| + | <br><br><b>Result:</b> We were able to visualize thylakoids directly bound to the nitrocellulose membrane based on the autofluorescence of chlorophyll pigment in the photosystems. However, no significant binding of thylakoids was observed at regions of the membrane that were pre-spotted with the candidate PSII-binding aptamer. | ||
| + | |||
| + | <br><br><center><img width="700" height="1000" src="https://static.igem.org/mediawiki/parts/3/30/T--Pretoria_UP--Thylakoid_binding.jpg"></center> | ||
| + | |||
| + | <p style="font-size:14px !important;text-align: center">Confocal micrograph of thylakoid binding to candidate DNA aptamers. Positive control: thylakoids bound directly to nitrocellulose membrane. Random region: background binding of thylakoids to blocked membrane. Aptamer: level of thylakoid binding to membrane-bound candidate DNA aptamer. Negative control: level of thylakoid binding to membrane-bound random oligo. | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | <div class="section section-we-are-1"> | ||
| + | <div class="text-area"> | ||
| + | <div class="container"> | ||
| + | <div class="row"> | ||
| + | <div class="title"> | ||
| + | <p class = "h2WM">Development of a graphene-binding DNA aptamer</p> | ||
| + | </div> | ||
| + | <div class="col-sm-12"> | ||
| + | <p style="text-align:left;"> | ||
| + | |||
| + | <div class="section section-we-are-1"> | ||
| + | <div class="text-area"> | ||
| + | <div class="container"> | ||
| + | <div class="row"> | ||
| + | <div class="title"> | ||
| + | <p class = "h2WM">Synthesis, cloning and expression of <i>Eucalyptus grandis</i> laccases</p> | ||
| + | </div> | ||
| + | <div class="col-sm-12"> | ||
| + | <p style="text-align:left;"> | ||
| + | <br>Here, we chose a rapid cell-free expression system that makes use of the SP6 phage promoter and does not require a transcriptional terminator. We searched for <i>Eucalyptus grandis</i> homologs of LAC4 and LAC17, two <i>Arabidopsis thaliana</i> laccase proteins known to reduce phenolic compounds much like the laccases from Trametes versicolor traditionally used in PBECs (Berthet et al. 2011). | ||
<br><br>Using BLASTP, we identified a number of laccase homologs in the <i>E. grandis</i> genome hosted on Phytozome (phytozome.jgi.doe.gov/pz/portal.html). We used gene expression data from EucGenIE (www.eucgenie.org; Hefer et al. 2011) to prioritise four laccase candidates based on high expression, especially in developing wood where these proteins are required for the polymerisation of monolignol substrates into lignin. | <br><br>Using BLASTP, we identified a number of laccase homologs in the <i>E. grandis</i> genome hosted on Phytozome (phytozome.jgi.doe.gov/pz/portal.html). We used gene expression data from EucGenIE (www.eucgenie.org; Hefer et al. 2011) to prioritise four laccase candidates based on high expression, especially in developing wood where these proteins are required for the polymerisation of monolignol substrates into lignin. | ||
| − | <br><br>Four Laccase proteins from <i>Eucalyptus grandis</i> were synthesized | + | <br><br>Four Laccase proteins from <i>Eucalyptus grandis</i> were synthesized: |
<br><br>Eucgr.A01282<br>Eucgr.F02641<br>Eucgr.B02797<br>Eucgr.G03098 | <br><br>Eucgr.A01282<br>Eucgr.F02641<br>Eucgr.B02797<br>Eucgr.G03098 | ||
| − | <br><br>Two of the laccases were cloned into pSB1C3 and submitted as new parts. | + | <br><br>Two of the laccases were cloned into pSB1C3 and submitted as new parts (see our parts page for more information). |
<br><br>For expression, an SP6 promoter was added for Phage SP6 RNA Polymerase to transcribe and translate the genes in vitro by making use of an SP6 in vitro expression kit (Promega TNT SP6 Coupled Wheat Germ Extract System). The advantage of this is that expression is fast, easy and does not require a T7 transcription termination sequence. | <br><br>For expression, an SP6 promoter was added for Phage SP6 RNA Polymerase to transcribe and translate the genes in vitro by making use of an SP6 in vitro expression kit (Promega TNT SP6 Coupled Wheat Germ Extract System). The advantage of this is that expression is fast, easy and does not require a T7 transcription termination sequence. | ||
| Line 77: | Line 157: | ||
</div><!--section section-we-are-1--> | </div><!--section section-we-are-1--> | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<br> | <br> | ||
</p> | </p> | ||
Revision as of 22:49, 19 October 2016
Project Results We divided our synthetic biology work into three sub-projects:
Development of a PSII-binding DNA aptamer
Designing a DNA aptamer for Photosystem II (PSII)
B-factor testing of Photosystem II (PSII) using Pymol. Red shading indicates higher mobility; blue shading denotes higher rigidity.
Figure depicting the core PSII components, with different colours representing distinct subunits of the complex. Pink, CP47; Cyan, CP43; green, D1; yellow, D2. Image drawn in Pymol.
Figure depicting the predicted binding of the single stranded DNA aptamer candidate designed by the MAWS software (5' TACGTTAT 3'). The aptamer was predicted to bind to the stromal side of the CP47 subunit. Image drawn in Pymol.
Isolation of thylakoids
Thylakoid extractions from spinach leaves. (Left) Percoll gradient-mediated purification. The top green layer contains a mixture of thylakoid membranes and broken chloroplasts, while the bottom layer contains pure intact chloroplasts. (Right) The resulting product of the simplified extraction.
Assessment of thylakoid binding by candidate aptamer
Confocal micrograph of thylakoid binding to candidate DNA aptamers. Positive control: thylakoids bound directly to nitrocellulose membrane. Random region: background binding of thylakoids to blocked membrane. Aptamer: level of thylakoid binding to membrane-bound candidate DNA aptamer. Negative control: level of thylakoid binding to membrane-bound random oligo.
Development of a graphene-binding DNA aptamer
Synthesis, cloning and expression of Eucalyptus grandis laccases
Sequencing result of cloned SP6 promoter parts. Four of six clones sequenced produced chromatograms agreeing with the reference sequence.
References
1. Development of a PSII-binding DNA aptamer (based on in silico prediction)
2. Development of a graphene-binding DNA aptamer (using SELEX)
3. Characterisation of novel laccase enzymes that might be used to reduce oxygen and hydrogen back to water on the cathode electrode of our PBEC.

We chose PSII rather than Photosystem I (PSI) as our system of choice as it only requires water for energy generation whereas PSI requires alternative co-factors. We then analysed one dimer of the PSII using Pymol and started off with B-factor analysis. B-factor refers to the displacement of atoms from their mean position in a crystal structure (Trueblood et al., 1996) . Using this analysis tool, we were able to determine which regions of the PSII complex are the most rigid and structurally stable and hence most suitable for aptamer-mediated targeting.
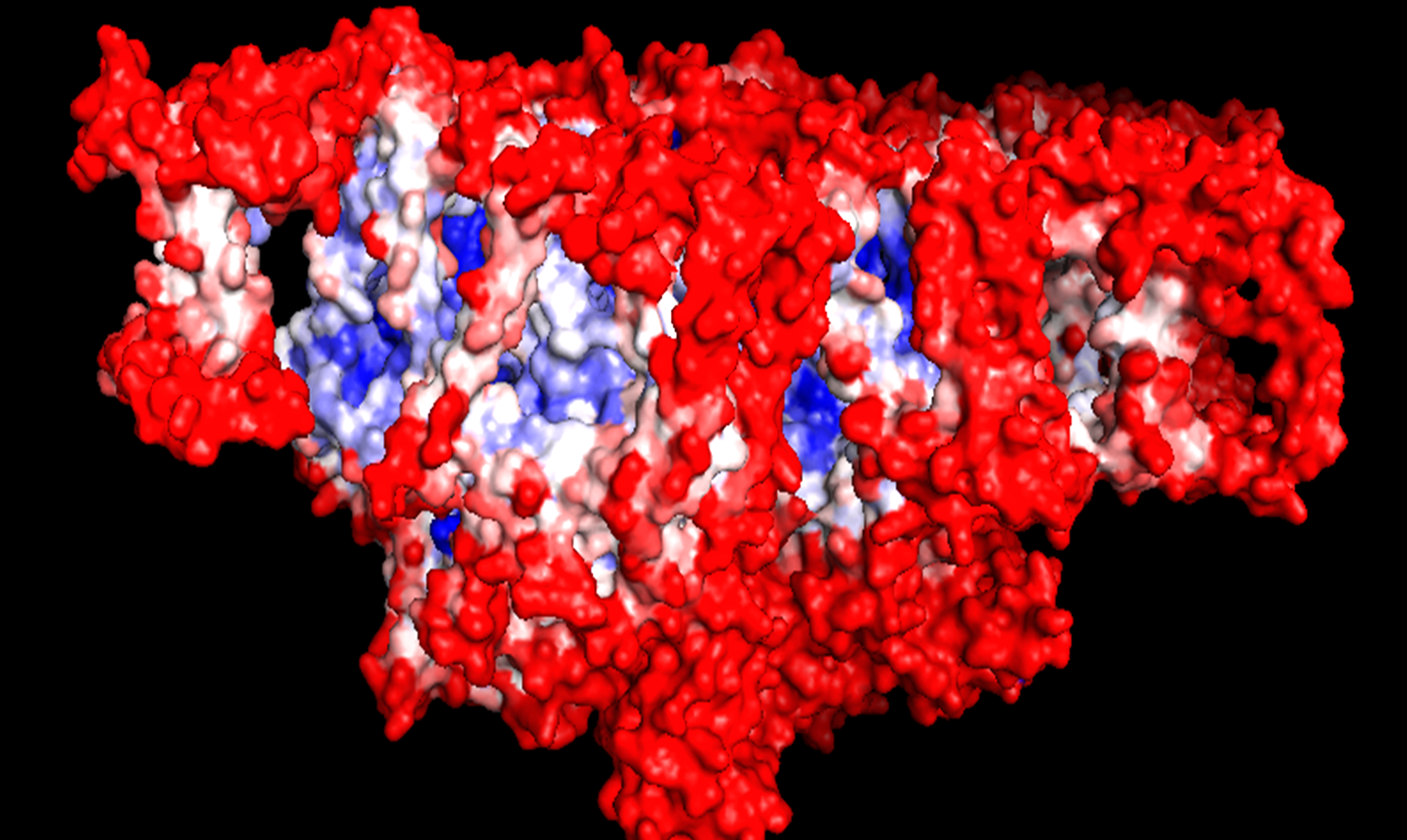
The result of the B-factor analysis revealed two proteins in the complex that were structurally more stable than the rest, namely CP47 and CP43. We decided to target the CP43 and CP47 subunits of the light-harvesting complex (LHC) of PSII to which the one side of the aptamers can bind. This protein is suitable as it is exposed and on the stromal side of the thylakoid membranes (which is important because electrons can more easily be abstracted on the stromal side). These proteins form the core subunits of the PSII complex and we determined them to be the most stable. We used PDF files from a recent crystal structure of the PSII complex (Wei et al., 2016) as the basis for aptamer design. iGEM team Heidelberg (2015) used their designed MAWS (Making Aptamers Without SELEX) software to design an aptamer sequence which has specifically bind to CP43 and CP47 subunits of PSII.
We then removed all non-target proteins and cofactors from the PSII PDB file using Pymol, so that we were left with CP43 and CP47 subunits alone. This file was then sent to Heidelberg 2015 iGEM team who used it as a template for aptamer targeting using their MAWS software. At first the PSII protein complex was too large for the program to compute a prediction, so we reduced the size of the PDB file to target only the stromal side of CP47. This was used as input for which was then ran through the MAWS software to test for the best aptamer oligo sequence. The predicted aptamer candidate had the sequence 5' TACGTTAT 3'.


Thylakoid extraction was carried out on store-bought spinach, using a protocol designed to extract and isolate intact chloroplasts from leaves, which we derived from Lang et al. (2011), Nagahashi et al. (1985), Rödiger et al. (2010) and Shiraya et al. (2014) as described below . The first protocol used involved grinding frozen tissue with a homogenizer in liquid nitrogen. A volume of 30ml of a “homogenization buffer” was added to 2 - 5 g of tissue, vortexed for 30 seconds and filtered through two layers of 100 µm nylon mesh, followed by two layers of 60 µm mesh. The filtrate was centrifuged at 200 x g for 4 minutes at 4°C, and the supernatant was then centrifuged for 10 minutes at 1500 x g at 4°C. The resulting pellet contained the chloroplasts. The pellet was gently resuspended in 3ml of “resuspension buffer”. Next the plastid suspension was slowly layered onto a discontinuous Percoll density gradient solution, containing equal volumes (9ml) of 85%, 40% and 10% Percoll solutions. The Percoll density gradient solution was then centrifuged at 5000 x g for 45 min at 4°C, after this centrifugation, intact chloroplasts were located on the interface of the 40/85 Percoll and the 10/40 interface contained broken chloroplasts and thylakoid membranes. Both fractions were gently transferred to separate falcon tubes, to which 3 volumes of “wash buffer” was added and then gently washed by centrifugation at 1500 x g for 10 min at 4°C (repeated once). The pellet was then resuspended in wash buffer and 50% glycerol solution for storage.
This method extracted extremely pure, intact chloroplasts, but in small yields. Because we only required thylakoid membranes, not intact chloroplasts, separation using a Percoll gradient was not necessary. Furthermore, the protocol used small volumes of material and therefore resulted in a small amount of product. We therefore simplified and scaled up the protocol for crude thylakoid extractions:
- A larger amount of material (5 - 10 g) was used
- Separation via the percoll density gradient was omitted
- The first crude pellet containing the chloroplasts and thylakoids was resuspended in the “wash buffer” and then sonicated to disrupt the chloroplast membrane and therefore increase the amount of free thylakoids in the solution.
The recipes of the buffers used may be seen in the tables below:





1. We synthesized the predicted PSII-binding aptamer (5' TACGTTAT 3') with an extra "foot" of random nucleic acids of 25-nt in length to give it a foothold to a nitrocellulose membrane. As a negative control, we synthesized a random oligo of the same length. (Reagents/materials used:
- Aptamers (N)25TACGTTAT: Stock (100uM) and working solution (10uM)
Random sequence as negative control
Nitrocellulose membrane
Thylakoids
Cherring testis DNA (1ul diluted in 20ml TBS)
TBS:
5% (m/v) non-fat milk in TBS
Procedure:
2. We made serial dilutions of the aptamers and negative control sequences from the 10 uM working solution: undiluted, 1/10, 1/100, 1/500, 1/1000.
3. The nitrocellulose membrane was cut to the desired size, marked and spotted with 3 ul of aptamer solution. As a positive control, the thylakoid extraction was spotted directly onto the membrane.
4. Non-specific sites were blocked by incubating it with the herring testis DNA for 30 min at 37°C followed by three wash steps with TBS (50 mM Tris-HCl pH 7.5, 150 mM NaCl).
5. The blot was further blocked with 5% m/v nonfat milk solution followed by washing three times with TBS.
6. The membrane was incubated in the thylakoid solution (diluted in TBS containing protease-inhibitor) on ice for 2 hours. It was then washed 3 times for 5 min with TBS at 4°C to remove any unbound thylakoids.
7. The blots were imaged with a Carl Zeiss LSM 5 confocal microscope, excitation wavelength 395 nm, to observe chlorophyll autofluorescence of the bound thylakoids.
Result: We were able to visualize thylakoids directly bound to the nitrocellulose membrane based on the autofluorescence of chlorophyll pigment in the photosystems. However, no significant binding of thylakoids was observed at regions of the membrane that were pre-spotted with the candidate PSII-binding aptamer.

Here, we chose a rapid cell-free expression system that makes use of the SP6 phage promoter and does not require a transcriptional terminator. We searched for Eucalyptus grandis homologs of LAC4 and LAC17, two Arabidopsis thaliana laccase proteins known to reduce phenolic compounds much like the laccases from Trametes versicolor traditionally used in PBECs (Berthet et al. 2011).
Using BLASTP, we identified a number of laccase homologs in the E. grandis genome hosted on Phytozome (phytozome.jgi.doe.gov/pz/portal.html). We used gene expression data from EucGenIE (www.eucgenie.org; Hefer et al. 2011) to prioritise four laccase candidates based on high expression, especially in developing wood where these proteins are required for the polymerisation of monolignol substrates into lignin.
Four Laccase proteins from Eucalyptus grandis were synthesized:
Eucgr.A01282
Eucgr.F02641
Eucgr.B02797
Eucgr.G03098
Two of the laccases were cloned into pSB1C3 and submitted as new parts (see our parts page for more information).
For expression, an SP6 promoter was added for Phage SP6 RNA Polymerase to transcribe and translate the genes in vitro by making use of an SP6 in vitro expression kit (Promega TNT SP6 Coupled Wheat Germ Extract System). The advantage of this is that expression is fast, easy and does not require a T7 transcription termination sequence.
Development of SP6 promoter as standard part
Two self-annealing oligos were synthesized to form a double-stranded SP6 promoter flanked by the RFC10 prefix and suffix sequences.
Oligo 1: 5’ CGCGAATTCGCGGCCGCTTCTAGAGATTTAGGTGACAC 3’
Oligo 2: 5’ CGCCTGCAGCGGCCGCTACTAGTACTATAGTGTCACCTAAA 3’
These oligos were allowed to annual at room temperature and then extended with Bsu DNA polymerase, large fragment (NEB) at 37C for 30 min. The resulting fragment was digested with EcoRI and PstI, cloned into pSB1C3, sequenced and submitted as part BBa_K2037000.
To test the functionality of this promoter as a standard part with RFC10 prefix and suffix sequence, we appended it to the mRFP fragment (BBa_J04450) for in vitro expression

Berthet, S., Demont-Caulet, N., Pollet, B., Bidzinski, P., Cézard, L., Bris, P.L., Borrega, N., Hervé, J., Blondet, E., Balzergue, S., Lapierre, C., Jouanin, L. (2011). Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. The Plant Cell 23: 1124-1137
Hefer, C., Mizrachi, E., Joubert, F., Myburg, A. (2011). The Eucalyptus genome integrative explorer (EucGenIE): a resource for Eucalyptus genomics and transcriptomics. BMC Proceedings 5: O49
Lang, E.G., Mueller, S.J., Hoernstein, S.N., Porankiewicz-Asplund, J., Vervliet-Scheebaum, M., Reski, R. (2011). Simultaneous isolation of pure and intact chloroplasts and mitochondria from moss as the basis for sub-cellular proteomics. Plant cell reports 30: 205-215.
Nagahashi, G., 1985. The marker concept in cell fractionation, In: Cell Components. Springer, pp. 66-84.
Rödiger, A., Baudisch, B., Klösgen, R.B. (2010). Simultaneous isolation of intact mitochondria and chloroplasts from a single pulping of plant tissue. Journal of plant physiology 167: 620-624.
Shiraya, T., Kaneko, K., Mitsui, T. (2014). Quantitative Proteomic Analysis of Intact Plastids. Plant Proteomics: Methods and Protocols 469-480.
Wei, X., Su, X., Cao, P., Liu, X., Chang, W., Li, M., Zhang, X., Liu, Z. (2016). Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 534:69–74.

