
Sleep occupies on average nearly a third of our lives. It does not only help us to regain strength, it is also essential for our brain development and to ensure many metabolic functions. It involves different brain mechanisms regulating the day/night rhythm, our daily sleep time and its quality. Sleep occurs in several stages and each of them is characterized by a different level of brain and muscle activity. Limited sleep time exposes people to different problems such as reduced vigilance, impaired learning and obesity. In the best of worlds, a person sleeps well, recovers from the day's events, and feels in top shape when waking up the next morning. However, most people do not have restorative nights caused by sleep disorders or other diseases. These can be temporary (a migraine that will keep us up one night or during the time adaptation when you have a jet lag) or last longer (lack of sleep due to anxiety provoked by exams), but these disorders can worsen and lead to new, aggravated symptoms. This is the case for people suffering from chronic insomnia or narcolepsy. Circadian rhythm sleep disorder is a family of sleep disorders due to the desynchronisation of the circadian rhythme or the "body clock". The circadian rhythmes time eating, sleeping, hormone regulation, body temperature variations ect. There are several distinct types of Circadian rhythm sleep disorders: the jet lag, the Advanced or Delayed sleep phase disorder and the Shift work sleep disorder.
Insomnia is a common sleep disorder defined by night time and daytime symptoms. Night time symptoms include persistent difficulties to fall and/or stay asleep and/or non-restorative sleep. Daytime symptoms of insomnia can include diminished sense of well-being, compromised functioning such as difficulties with concentration and memory, fatigue, concerns and worries about sleep. The diagnosis is made when the symptoms persist for at least 1 month and insomnia is considered chronic if it persists for at least 6 months [3]. Narcolepsy (hypersomnia) is a rare disease that affects 2 people per 10 000 and usually begins in adolescence. It is an inability to maintain the awakening more than two hours causing uncontrollable sleepiness. People suffering from this disease are also subject to cataplexy attacks resulting in a sudden muscle relaxation and hallucinations that are actually daydreams. These crises are often triggered by a positive emotion like laughter. This is due to a loss of orexin neurons (also known as hypocretin) by a probably autoimmune mechanism [1]. Concerning the temporary sleep troubles, alternative therapies using no medication can have positive effects on some disorders or their symptoms. See our page Integrated Human Practices to see more about the therapies that we tested for you! For insomnia, it can be treated using non-pharmacological or pharmacological methods or a combination of both. It exists a specific therapy for insomnia called cognitive behavioral therapy, a specific psychotherapeutic approach with variants for treating different mental conditions such as depression, anxiety and eating disorders. When insomnia is experienced in the context of another disorder, such as depression, general psychotherapy might be effective in helping with depression but not be as helpful with the insomnia. Delta sleep-inducing peptide (DSIP) is a nonapeptide with a unique amino-acid sequence. Its aminoacid sequence is Trp-Ala-Gly-Gly-Asp-Ala-Ser-Gly-Glu and has a molecular weight of 849 dalton. However, the gene which produce it is unknown [4]. Caenorhabditis elegans is a nematode, which is commonly used as a model for eukaryotic genetic studies due to its small size (about 1mm long) and its rapid life cycle. The experimental strengths and the similarities between the cellular and molecular processes present in C. elegans and other animals across evolutionary time (metabolism, organelle structure and function, gene regulation, protein biology, etc.) have made it an excellent organism with which to study general metazoan biology. At least 38% of the C. elegans protein-coding genes have predicted orthologs in the human genome (Shaye and Greenwald 2011), 60-80% of human genes have an ortholog in the C. elegans genome (Kaletta and Hengartner, 2006), and 40% of genes known to be associated with human diseases have clear orthologs in the C. elegans genome (Culetto and Satelle, 2000). Thus, many discoveries in C. elegans have relevance to the study of human health and disease. In addition, because the animal has an invariant number of somatic cells (1031 for males), researchers have been able to track the fate of every cell between fertilization and adulthood to generate a complete cell lineage. In particular, all 302 neurons have been identified to create a map of C. elegans’s nervous system (White et al. 1986) which is used in the field of neurobiology. Today, C. elegans is actively studied in over a thousand laboratories worldwide with over 1200 C. elegans research articles published each year for the last 5 years. After fertilization, the C. elegans embryos are usually retained within the hermaphrodite until about the 24-cell stage at which time they are laid. The hermaphrodite embryo hatches with 558 nuclei and becomes a first stage (L1) larva. The animals begin to eat and develop through four larval stages (L1-L4). The L1 stage is ~16 hr long; the other stages are ~12 hr long. Each stage ends with a sleep-like period of inactivity called lethargus (Raizen et al. 2008) in which a new cuticle (outer collagenous layer) is made. Lethargus ends with the molting of the old cuticle. Approximately 12 hr after the L4 molt, adult hermaphrodites begin producing progeny for a period of 2-3 days until they have utilized all of their self-produced sperm. After the reproductive period, hermaphrodites can live several more weeks before dying of senescence. C. elegans has two states that fulfill all the behavioral criteria for sleep:
For our first approach, we decided to use C. elegans as a model for sleep and create a genetic system to regulate sleep cycles. In order to do this, we chose two neuropeptides which are central to the regulation of sleep: NLP-22 and PDF. It has been shown that the neuropeptide NLP-22 is a regulator of C. elegans sleep state during lethargus and that it is regulated by LIN-42, an orthologue to the core circadian protein PERIOD. [9] This similarity between C. elegans and mammalian sleep patterns reinforces the fact that C. elegans is a suitable model for sleep. NLP-22 is only expressed in the 2 RIA interneurons of C. elegans and forced expression of NLP-22 during active phases causes quiescence of feeding and locomotion. iGEM Bordeaux chose to use this neuropeptide in our construction in order to induce sleep in response to light simulation. In order to wake our C. elegans, we chose to use the neuropeptide PDF (pigment dispersing factor) which has been shown to be involved in wake behaviour in C. elegans and in promoting morning behaviour in Drosophilia. [10]. By using both these neuropeptides, iGEM Bordeaux hopes to create a genetic switch to control sleep in C. elegans with different light waves. Once we found the neuropeptides which we would express in C. elegans we needed to create our genetic switch using photo inducible proteins. An interesting system has been described in Neurospora crassa [11] which we decided to adapt for C. elegans. Naturally the white collar complex (WC1-WC2) assembles through the PAS domains present on both proteins. The zinc fingers allow the complex to bind to specific regions in DNA and the LOV domains allows the complex to bind FAD and transform light into mechanical energy.
It has been shown that the white collar complex binds to three specific regions on the frequency (FRQ) promoter (pFRQ). They are shown on figure 2B and are respectively the dLRE, pLRE, and aLRE. The consensus binding sequence is shown on figure 2A. However, it has been shown that the promoter can activate transcription when it is reduced to 300 base pairs. In order to simplify our construction, we chose to use this reduced version of pFRQ and not it’s full sequence.
Lastly a constitutive promoter present in C. elegans’s neurons (Unc 119) was used in order to express the white collar complex in all neurons. Theoretically, the complex should be synthetized but only activated in the presence of blue light. The complex will then change conformation allowing it to bind to the pFRQ promoter and activating the transcription of NLP-22. C. elegans should then fall asleep.
A similar construction using a second photoinducible system in plants (PHY-B and PIF 3) was imagined but not put in place due to time constraints. Theoretically, these two proteins assemble and activate the GAL 1 promoter with red light. With a similar system as described above, this would allow us to express the pigment dispersing factor (PDF) neuropeptide which would wake our nematode up in the presence of red light. Goals: On a first hand, we will synthesize FRQ promoter and NLP22 gene thanks to IDT. On a second hand, WC1 and WC2 will be extracted from Neurospora tetrasperma’s genome and each promoter used will be extracted from Caenorhabditis elegans’s genome (pRPS-27, pUNC-119, pXBP1, pmyo2 and pmyo3).
A part will be specially created in order to be after each construction: 2Apeptide::GFP (BBa_S05331). This construction will permit us to characterize each Biobrick.
Based on CRISPR-CAS9 tool, the second approach aims to bring a modified DNMT specifically to any CGI promoters within the genome of eukaryotes. This will be achieved by fusing a DNAse-dead Cas9 (dCas9) to the DNMT enzymatic core. It will be recruited in a sequence-specific way via sequence homology of a single guide (sg) RNA with sites chosen by the experimentator. This tool will first test the construction in C. elegans, which is an organism with a free-methylated cytosine genome [12]. We will be able to evaluate the efficiency and the specificity of our system without any background. Next, we will assess the EpiCRISPR range of methylation by targeting HOT (highly occupied target) regions known to be C. elegans’ orthologs of vertebrate CGI. [13]. With several DNMT3a/DNMT3l spread along the HOT region, our system is probably prone to expand the potential range of methylation of a CpG promoter. Our project is built on three key players: a dCas9, DNMT3a and DNMT3l. To achieve better results with EpiCRISPR, our team plans three strategies: That is why this first approach acts as a test whereas we place our hopes in the second strategy: Goals: We will confirm the expression and the activity of EpiCRISPR by in vitro techniques: immuno-precipitation with antibodies directed against dSpCas9 and DNMT3a will assure us that the complex is formed into the cell. To check the emerging of 5mC in C.elegans’ genome, we will see if antibodies directed against 6mA (naturally present in this organism) and 5mC bind to its DNA. This is the Dot Blot method.

1. The problem: Sleep disorders
Sleep
We all have an internal biological clock that regulates our 24-hour sleep/awake cycle, also known as our circadian rhythm. Light is the primary factor that influences our circadian rhythm. When the sun comes up in the morning, the brain “tells” our body that it is time to wake up. At night, when there is less light, our brain triggers the release of melatonin , a hormone that makes us sleepy.
When one’s circadian rhythm is disrupted or thrown off, they may feel disoriented and sleepy at inconvenient times. The disruption of the circadian rhythm has been linked to a variety of sleep problems and disorders, including insomnia, jet lag, and shift work sleep difficulties. An abnormal circadian rhythm can also be implicated in depression or bipolar disorder.About 20 to 30% of the french population complains of sleep disorders with 15-20% suffering from moderate insomnia and 10% suffering from severe insomnia [1].
Sleep disorders and diseases
Circadian dysrhythmia
Insomnia
Narcolepsy
Alternative therapies and medications
There are a number of different medications which are used to manage insomnia. Properly used, they can be very beneficial. However, possible side effects should be carefully considered. The Food and Drug Administration (FDA) has approved certain medications for the treatment of insomnia. These are called hypnotic medications or sleep medications. One class of sleep medication is called benzodiazepines. Benzodiazepines are generally recommended for short-term use because tolerance and dependence can develop. In addition, some medications in this class can produce a "hangover" or grogginess the next day [3].
Concerning narcolepsy, there is no specific cure, but it is possible to manage the symptoms and minimise their impact on the daily life. In addition, this disease is known to have more than one underlying cause. Medications used to relieve sleepiness are all stimulants or wake promoting medications. Sleepiness is often improved with modafinil, amphetamines, or sodium oxybate. Cataplexy is often improved with antidepressants or sodium oxybate. However, people using high doses of these medications can become addicted and dependent, requiring higher doses of the drug and exhibiting signs of withdrawal when the drug is discontinued.
There is a continuing research on Narcolepsy, how the sleep/wake cycle works and new medications. Emerging treatments being investigated for Narcolepsy include hypocretin replacement, hypocretin gene therapy and immunotherapy, but further research is needed before any may be available in clinical practice.Our solution : DSIP
Some studies have demonstrated that DSIP is present in the hypothalamus and targets multiple sites including some within the brain stem.
The link between DSIP and sleep hasn't been really characterized because of the lack of isolation of the DSIP gene, peptide and possible related receptor but this peptide appears to promote sleep.
DSIP is unusual because it can cross the blood-brain barrier and is absorbed from the gut without being denatured by enzymes [5]. Since the discovery of DSIP, a number of studies have been undertaken to test the hypothesis that this peptide may be the principal endogenous sleep factor. It is reported for increasing the pressure to sleep in human [6]. Its ability to induce delta-wave sleep led to its use in insomnia treatment. It has a greater activity when sleep is disturbed, has a minimal effects in healthy subjects and restores the circadian rythms. It also increases alertness during awake cycles with an improved stress tolerance.
A dose of DSIP given during the day improves sleep on the next night and for several nights thereafter. In some studies, administration of DSIP has reduced narcolepsy effects and normalized disturbed sleeping patterns. The peptide is now known to have multifunctional regulatory properties and numerous studies have confirmed its stress-protective and adaptive activity.
DSIP has non-sleep effects on stress, obesity and drug addiction :
Our first goal aims to test this peptide on the model Caenorhabditis elegans in order to see these effects on its sleep.
2. Caenorhabditis elegans
Why use this model?
Developmental cycle of Caenorhabditis elegans [7]
How does Caenorhabditis elegans sleep? [8]
SIS occurs following exposure to environmental stimuli that cause cellular stress, and its duration depends on the severity of the stressor.
For our project, we decided to focus on developmentally timed sleep and on how to induce or repress DTS in our model organism.Sleep with EpiC elegans aims to study the sleep regulation of Caenorhabditis elegans. For that, we have imagined two ways for controling the sleep regulation in C. elegans.
3. Studying the sleep regulation in Caenorhabditis elegans
The photo inducible system

The creation of the photo inducible system

Both sub units (WC-1 and WC-2) assemble through their PAS domains and the zinc finger complex allows the complex to bind to specific regions of DNA (for example specific regions of the FRQ promoter: pFRQ). The LOV domains allows the complex to bind FAD and transform light into mechanical energy.

The new tool : EpiCRISPR

Design of the tool
Before us, Albert Jeltsch’s team has engineered a single chain (sc) fusion protein of catalytic core of murine Dnmt3a and activator domain of Dnmt3l which has a 7-fold increased activity in vitro [14]. With this fusion protein fused to a TALEN, another team [15] managed to methylate about 20% of the targeted CpG promoter and to reduce by one third the targeted gene transcription level.

The fusion protein dCas9 ::DNMT3a ::DNMT3l is recruited by the guide RNA to a specific locus to drive cytosine methylation

dCas9 provides several epitopes which are recognized by a fusion protein scFv ::DNMT3a ::DNMT3l. The DNA methylase activity is amplified. However, this fusion protein can be led to any cytosine of the genome because of the natural affinity of the enzyme for its substrat.
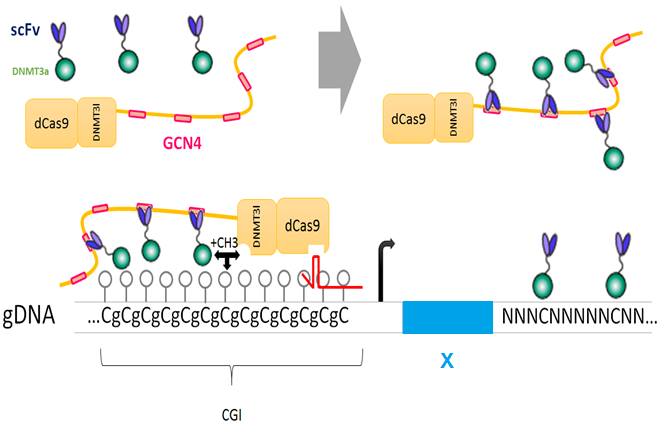
The fusion protein dCas9 ::DNMT3l provides several epitopes which are recognized by a fusion protein scFv ::DNMT3a. The DNA methylase is active only when DNMT3a and DNMT3l are gathered, it means in the locus targeted by the guide RNA
To see the effect on gene expression, we will target a plasmid with a SET-16 promoter and a GFP underneath. We expect the worm to lose its fluorescence only when EpiCRISPR is micro-injected. Whether cytosine methylation of a promoter in the worm results in a decreased transcription level of downstream genes (or not) will be a new discovery.
EpiCRISPR would then need to be further tested in vertebrates in order to prove its impact on down regulation of gene expression without DNA modification.References


