
Results
Shuttle Vector
(I) Introduction
In order to use photo-inactivated Leishmania as a safe carrier to deliver specific antigens to the APCs for T and B cell stimulation, we designed an E. coli-Leishmania shuttle vector for antigen expression in Leishmania.
A shuttle vector is a vector constructed so that it can reproduce in two different host species. The main purpose of these vectors is that they can be quickly amplified in E. coli and then manipulated in another organism, such as Leishmania. Here we designed an E.coli-Leishmania shuttle vector constructed under biobrick standards to provide a standardized shuttle vector for our own experiment and for others’ future application.
A shuttle vector is a vector constructed so that it can reproduce in two different host species. The main purpose of these vectors is that they can be quickly amplified in E. coli and then manipulated in another organism, such as Leishmania. Here we designed an E.coli-Leishmania shuttle vector constructed under biobrick standards to provide a standardized shuttle vector for our own experiment and for others’ future application.
(II) Design
Leishmania shuttle vector
In Leishmania genome, p36 and nagt genes are separated by an intergenic region of ~2300 bp, which contains stage-independent splicing sites for 5’ miniexon and 3’ polyA addition. Spliceosome-mediated trans-splicing of chromosome-length polycistronic transcripts is expected to produce mature p36 and nagt mRNAs, each flanked with 5' and 3' UTRs. These UTRs are thought to stabilize the p36 and nagt mRNAs, accounting for their constitutive expression in both Leishmania stages. According to these features, we decided to make a Leishmania shuttle vector in compliance with the biobrick format.

The first coding region, p36, is replaced with hygromycin resistant gene as a selection marker in leishmania, and combined with 5’UTR as a biobrick part, since the 5’UTR may contains promoter and ribosome binding site and other important functions in leishmania. As for the second coding region, NAGT, is allowed to be replaced with any protein we want leishmania to carry. In our project, we want to put hemagglutinin (HA) of H1N1 and ovalbumin (OVA) into the site to prove our concept. The 3’UTR of the sequence was designed to become a terminator part while the 2300 intrinsic sequence was also needed to regulate the expression of the protein in the shuttle vector.
The leishmania gene would be put into pSB1C3 to become a shuttle vector that could quickly proliferate in e.coli and express proteins in leishmania. There are 3 advantages of our shuttle vector. First, it contains two coding regions originally, so our shuttle vector will be a good choice to express two proteins at the same time. Second, the 2300 intrinsic sequence in our shuttle vector has many stage-independent splicing sites to cut the polycistronic RNA into two transcripts in any stage of leishmania. The third is that, through the biobrick design, we can build a standard shuttle vector for leishmania and also introduce a new animal model into iGEM competition.

Transfect the pSB1C3-5'HYG-OVA-3'UTR and pSB1C3-5'HYG-HA-3'UTR into Leishmania by electroporation
We used electroporation to transfect our constructs into Leishmania and tested the expression of the encoded proteins by western blot assay. The pSB1C3-5'HYG-OVA-3'UTR and pSB1C3-5'HYG-HA-3'UTR would be transfected into leishmania 12-DT strain by electroporation and compared with the wild-type 12-DT strain.
The parts-validating experiments by LacI inducible system
Since we are performing immunology experiments, it is very important that the antigen is correctly expressed. In order to prove the HA sequence we designed can be expressed correctly, we used BL21 competent cell to express the HA sequence and detect the protein by Western blot analysis.
Among the standard parts provided by iGEM, we chose BBa_J04500 as a promoter to induce production of protein. BBa_J04500 is a LacI inducible promoter with RBS, so it can be induced by IPTG to activate its promoter and start transcription.
Also, we used BBa_E0040, a GFP generator, as a positive control to confirm our experimental design in protein production.
In Leishmania genome, p36 and nagt genes are separated by an intergenic region of ~2300 bp, which contains stage-independent splicing sites for 5’ miniexon and 3’ polyA addition. Spliceosome-mediated trans-splicing of chromosome-length polycistronic transcripts is expected to produce mature p36 and nagt mRNAs, each flanked with 5' and 3' UTRs. These UTRs are thought to stabilize the p36 and nagt mRNAs, accounting for their constitutive expression in both Leishmania stages. According to these features, we decided to make a Leishmania shuttle vector in compliance with the biobrick format.

The first coding region, p36, is replaced with hygromycin resistant gene as a selection marker in leishmania, and combined with 5’UTR as a biobrick part, since the 5’UTR may contains promoter and ribosome binding site and other important functions in leishmania. As for the second coding region, NAGT, is allowed to be replaced with any protein we want leishmania to carry. In our project, we want to put hemagglutinin (HA) of H1N1 and ovalbumin (OVA) into the site to prove our concept. The 3’UTR of the sequence was designed to become a terminator part while the 2300 intrinsic sequence was also needed to regulate the expression of the protein in the shuttle vector.
The leishmania gene would be put into pSB1C3 to become a shuttle vector that could quickly proliferate in e.coli and express proteins in leishmania. There are 3 advantages of our shuttle vector. First, it contains two coding regions originally, so our shuttle vector will be a good choice to express two proteins at the same time. Second, the 2300 intrinsic sequence in our shuttle vector has many stage-independent splicing sites to cut the polycistronic RNA into two transcripts in any stage of leishmania. The third is that, through the biobrick design, we can build a standard shuttle vector for leishmania and also introduce a new animal model into iGEM competition.

Transfect the pSB1C3-5'HYG-OVA-3'UTR and pSB1C3-5'HYG-HA-3'UTR into Leishmania by electroporation
We used electroporation to transfect our constructs into Leishmania and tested the expression of the encoded proteins by western blot assay. The pSB1C3-5'HYG-OVA-3'UTR and pSB1C3-5'HYG-HA-3'UTR would be transfected into leishmania 12-DT strain by electroporation and compared with the wild-type 12-DT strain.
The parts-validating experiments by LacI inducible system
Since we are performing immunology experiments, it is very important that the antigen is correctly expressed. In order to prove the HA sequence we designed can be expressed correctly, we used BL21 competent cell to express the HA sequence and detect the protein by Western blot analysis.
Among the standard parts provided by iGEM, we chose BBa_J04500 as a promoter to induce production of protein. BBa_J04500 is a LacI inducible promoter with RBS, so it can be induced by IPTG to activate its promoter and start transcription.
Also, we used BBa_E0040, a GFP generator, as a positive control to confirm our experimental design in protein production.
(III) Result
The construction of pSB1C3-5’HYG-HA-3’UTR and pSB1C3-5’HYG-OVA-3’UTR
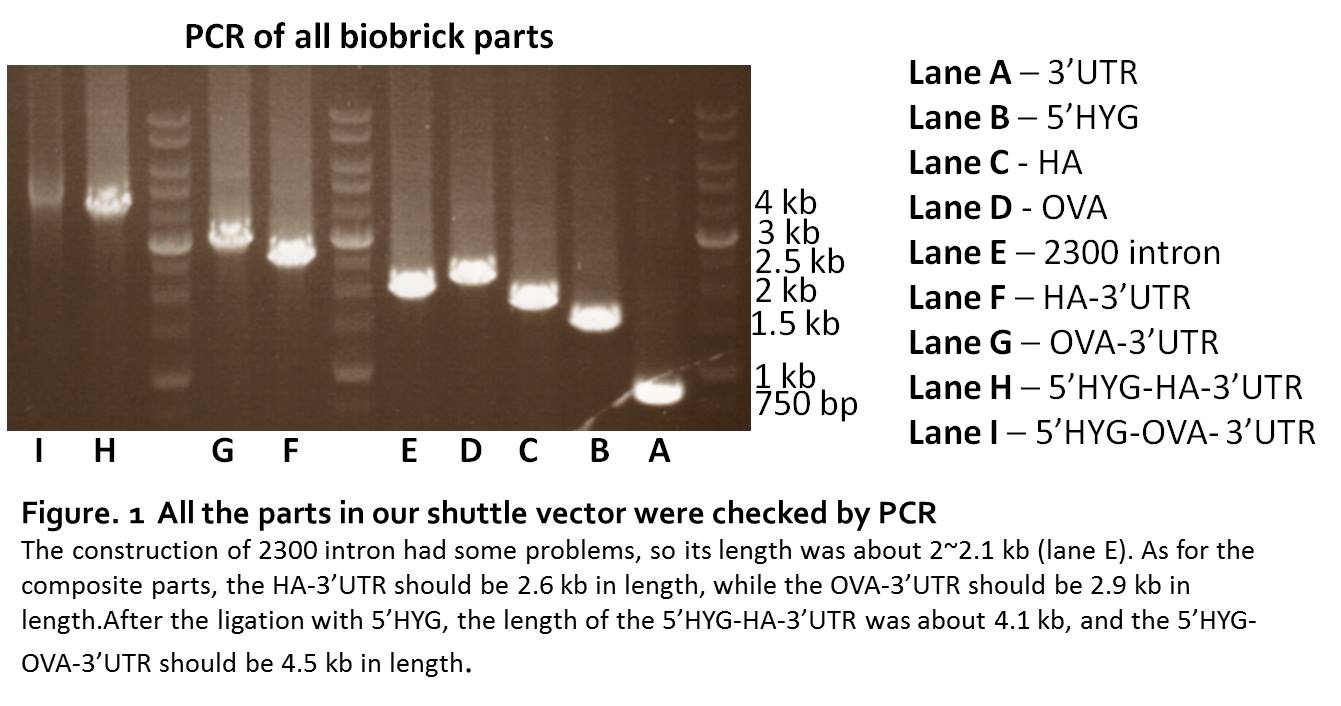
We synthesized the 5’HYG, 3’UTR, HA, OVA sequence directly by IDT. The synthesized sequence were digested and ligated to pSB1C3. The parts were checked by PCR. The length of 5’HYG was 1446 bp, HA was 1700 bp, OVA was 2098 bp and 3’UTR was 774 bp (Fig. 1, lane A.B.C.D). To build a workable leishmania shuttle vector, we generate two constructs, pSB1C3-5’HYG-HA-3’UTR and pSB1C3-5’HYG-OVA-3’UTR (Figure 1. lane F.G.H.I).
The construction of pSB1C3-2300intron
Since the 2300 intron contained too many CG pairs, it’s not allowed to synthesis. We used point mutation to change the nucleotide in the 2300 bp sequence, therefore, the sequence would be separated into 3 parts. Through the PCR, we could have these 3 parts amplified from p6.5 plasmid, then used the PCR-after-ligation strategy to construct the pSB1C3-2300intron part (Figure 1. lane E). Although the parts of 2300 intron could be proliferated by PCR, we were unable to ligate the 3 parts together. The sequencing results of the 2300 intron always lost the second part, no matter what strategy we used in the construction. So, it turned out that we couldn’t put the 2300 intrinsic region into the final construction of our shuttle vector. Therefore, we removed 2300 intron from our design.
Establishment of stable leishmania transfectants and test the protein expression
The pSB1C3-5'HYG-OVA-3'UTR was transfected into Leishmania 12-DT strain, and the transfectants were selected by hygromycin. After 20 days of hygromycin gradient selection, we obtained the hygromycin-resistant Leishmania transfectants (Figure 2. B, C). In the negative control group (Figure 2. A), we could see that almost all cells were dead and the shape of Leishmania was not normal. It showed that Leishmania transfectants were resistant to high concentration of hygromycin. The cell numbers increased normally just like the wild type 12-DT strain without hygromycin selection. And the viability and motility was also similar to wild type 12-DT strain. These results showed that the 5’HYG part could function in Leishmania. The function of 5’HYG was assayed more precisely in figure 3. The pattern showed that the pSB1C3-5'HYG-OVA-3'UTR leishmania transfectants can resist to hygromycin and grow normally. According to figure 2 and figure 3, we could indicate that the designed pSB1C3-5'HYG-OVA-3'UTR construction can express hygromycin resistant gene successfully.
We tried to verify the second encoded gene by western blot analysis, but can’t obtain an apparent band of OVA protein. It showed that the pSB1C3-5'HYG-OVA-3'UTR without the regulatory sequence of 2300 intron may not expressed the second encoded gene efficiently. The Leishmania transfected with pSB1C3-5'HYG-OVA-3'UTR were collected and used in in vivo experiment to test if inactivated OVA-expressing Leishmania could induce immune responses.
We also transfected pSB1C3-5'HYG-HA-3'UTR into leishmania, but the transfected leishmania couldn’t live well after hygromycin selection. However, the HA protein was able to express in BL21 by the lacI inducible system from our parts-validating experiments. The HA protein could be recognized by the HA antibody in western blot assay, which meant that the HA part could translate the right protein.

Transform pSB1C3-J04500-HA into BL21 to express desired gene.
For the pSB1C3-J04500-HA, their induction can be checked by Coomassie blue or Western blot analysis. We successfully recognize the HA protein from Western blot analysis (approximately 62.3kd) when induced by IPTG (Figure 4.).

Leijuvant
– the immune response of photo-inactivated leishmania as adjuvant in mice
(I) Introduction
To prove our concept, we tested the efficiency of the antibody immune response and T cell immune response of the photo-inactivated Leishmania as a vaccine adjuvant. Ovalbumin (OVA) has been commonly used as the antigen for testing the efficiency of antibody response and T cell activation in previous immunology experiments. Also, OVA is the only foreign antigen carried by Leishmania that has been shown to load the major histocompatibility complex class I molecules (MHC I) after phagocytosis by APCs, since the transgenic mutants of Leishmania is a new way to deliver antigens into antigen-presenting cells (APC). Thus, we want to use OVA in our in vivo test to validate our hypothesis of photo-inactivated Leishmania as an adjuvant. We co-injected OVA recombinant protein and photo-inactivated Leishmania that is genetically modified to present OVA protein into mouse.
Serum is collected every 5 days after the second injection to test the antibody immune response with Anti-OVA ELISA and further tested the T cell response with dissected splenic cell. The outcome will be compare to the of Alum adjuvant.
(II) Design
We subcutaneously co-injected leish-OVA (Leishmania expressing OVA) and OVA protein into mice and compare the outcome to the of Alum adjuvant with OVA protein as a positive control. We immunized the mice twice, the second boost will be injected on the 15th day after the first shot and after the second shot we will collect serum from the mice on the 5th 10th 13th day after. The serum will be tested for anti-OVA IgG1 and IgG2a as the antibody response and the cell-mediated immune response, respectively. We will dissect the spleen on the 10th day after the second boost and culture the splenic cells for 6 days. Culture supernatant will be tested for cytokines specific for T cell response.


(III) Result
The result of a particular IgG subclass secreted from B cells is determined by which type of T cell is induced. Proliferating helper T cells will develop into effector T cells and differentiate into two major subtypes, T helper 1(Th1) and T helper2(Th2). Th1 cells mainly promote cellular immune pathway which maximizes the killing efficacy of macrophages, the proliferation of cytotoxic cluster of differentiation 8 (CD8) T cells and stimulate the production of Immunoglobulin G 2a (IgG2a)[1]. Th2 cells dominantly facilitate humoral immune pathway, stimulating B-cells into proliferation and production of neutralizing antibodies such as Immunoglobulin G (IgG), Immunoglobulin M (IgM) and Immunoglobulin A (IgA) and Immunoglobulin E (IgE) antibodies.
Th1 can secrete cytokines, e.g. Interferon-γ (IFN-γ), to help stimulate other immune cells which give rise to the positive feedback. Therefore, promotes the Th1 profile. Th2 can secrete Interleukin 10 (IL-10) which down regulates the expression of Th1 cytokines but enhances B cell survival, proliferation, and antibody IgG1 production [2]. Therefore, higher IgG1/IgG2a ratio indicates increasing activity of the Th2-pathway.

Antibody titer of the serum samples collected from immunized mice were measured with anti-OVA IgG1 and IgG2a ELISA. The antibody titer of Alum+OVA group on the 25th day is set as 100%.
The IgG1 antibody titer increased drastically after the second boost on the 15th day. And the antibody titer reached to peak on the 25th day (Figure 2A.). Inactivated Leishmania can achieve over 60% of the antibody titer. As for OVA-expressing inactivated Leishmania, the antibody titer can reach to over 70% on the 25th day. The IgG2a antibody titer of inactivated Leishmania increased extremely on the 20th day and is significantly higher compare to Alum on the 25th day (Figure 2B.). The productions of IgG2a from inactivated Leishmania groups are much higher than Alum. Alum is able to induce a good Th2 response (IgG1), but it has little capacity to stimulate Th1 immune responses [3] and thus has a lower production of IgG2a. Inactivated Leishmania as the adjuvant has a higher tendency to stimulate Th1 immune responses than Alum.

The concentration of IL-10 and IFN γ released from splenocytes were measured by IL-10 and IFN γ ELISA (Figure 3.). The splenocytes were collected from spleens dissected form mice on the 25th day of immunization. The IL-10 secretion of inactivated Leishmania stimulated with OVA is significantly higher than the positive control (ConA). IL-10 secreted form Th2 may help the production of IgG1. As for the IFN γ, the secretion concentration was low and didn’t have a significant difference.

Due to the limited sample size, there wasn’t any significant difference between inactivated Leishmania only and inactivated OVA-expressing Leishmania. The OVA-expressing Leishmania wasn’t a stable transfectant cell line yet when we performed the in vivo test. Owing to the time limits, there was still 1/3 of the complete drug selection process remaining to select the stable tranfectants with high expression of the target antigen. Therefore, the result of injecting stable transfectants of inactivated OVA-expressing Leishmania may have a much higher antibody production.
We conclude that Leijuvant is a potential bi-pathway adjuvant that can stimulate both Th1 and Th2 immune responses.
Th1 can secrete cytokines, e.g. Interferon-γ (IFN-γ), to help stimulate other immune cells which give rise to the positive feedback. Therefore, promotes the Th1 profile. Th2 can secrete Interleukin 10 (IL-10) which down regulates the expression of Th1 cytokines but enhances B cell survival, proliferation, and antibody IgG1 production [2]. Therefore, higher IgG1/IgG2a ratio indicates increasing activity of the Th2-pathway.

Antibody titer of the serum samples collected from immunized mice were measured with anti-OVA IgG1 and IgG2a ELISA. The antibody titer of Alum+OVA group on the 25th day is set as 100%.
The IgG1 antibody titer increased drastically after the second boost on the 15th day. And the antibody titer reached to peak on the 25th day (Figure 2A.). Inactivated Leishmania can achieve over 60% of the antibody titer. As for OVA-expressing inactivated Leishmania, the antibody titer can reach to over 70% on the 25th day. The IgG2a antibody titer of inactivated Leishmania increased extremely on the 20th day and is significantly higher compare to Alum on the 25th day (Figure 2B.). The productions of IgG2a from inactivated Leishmania groups are much higher than Alum. Alum is able to induce a good Th2 response (IgG1), but it has little capacity to stimulate Th1 immune responses [3] and thus has a lower production of IgG2a. Inactivated Leishmania as the adjuvant has a higher tendency to stimulate Th1 immune responses than Alum.

The concentration of IL-10 and IFN γ released from splenocytes were measured by IL-10 and IFN γ ELISA (Figure 3.). The splenocytes were collected from spleens dissected form mice on the 25th day of immunization. The IL-10 secretion of inactivated Leishmania stimulated with OVA is significantly higher than the positive control (ConA). IL-10 secreted form Th2 may help the production of IgG1. As for the IFN γ, the secretion concentration was low and didn’t have a significant difference.

Due to the limited sample size, there wasn’t any significant difference between inactivated Leishmania only and inactivated OVA-expressing Leishmania. The OVA-expressing Leishmania wasn’t a stable transfectant cell line yet when we performed the in vivo test. Owing to the time limits, there was still 1/3 of the complete drug selection process remaining to select the stable tranfectants with high expression of the target antigen. Therefore, the result of injecting stable transfectants of inactivated OVA-expressing Leishmania may have a much higher antibody production.
We conclude that Leijuvant is a potential bi-pathway adjuvant that can stimulate both Th1 and Th2 immune responses.
MHC Presentation
(I) Introduction
The biobrick construct of E.coli-Leishmania shuttle vector is meant to express
the targeting antigen protein in Leishmania through amplification in E.coli and
transfection into Leishmania. Therefore, total size of the shuttle vector can
significantly affect the efficiency of transformation and transfection during the
procedure. To enhance the efficiency, we’ve tried to focus on shortening the
targeting antigen sequence which will then be sub-cloned into the shuttle vector. In
order to identify antigen sequence with the highest MHC binding affinity, researchers
have to utilize several bioinformatics tools to figure out or predict the protein
properties. In our project, we have generated an integrated protein information
website, McHug, to help users in searching for peptide sequences that can optimally
activate immune response. Other than providing users with all basic protein
information, McHug features the visualized interface which can transform loads of
numerical data into legible charts. The ultimate goal of McHug website is to mark all
the protein annotations on the given protein sequence and display the relative
immune properties such as MHC binding affinity in every position of the protein.
Selection of the most suitable sequence for MHC presentation can be easily
accomplished with McHug. The apllicability of McHug website was further tested in
vitro using OVA-loaded dendritic cells. MHC molecules were immunoprecipitated and
the associated peptide sequences were then analyzed by mass spectrometry. You can
learn faster and more about your targeting antigen while experiencing McHug.
(II) Design
Our in vitro experiment is to focus on immunoprecipitating MHCII and MHC I molecules to prepare LC/MS samples. The peptides on the MHC molecules will be isolated and sequenced to analyse the viability of our software, McHug. In figure 1, the schematic diagram of our co-culture and lysis system is described. Inactivated Leishmania was co-cultured with dendritic cells for several time points and be lysed by CHAPS buffer to collect the supernatant for further experiment. The ultimate goal of our in vitro experiment is to isolate the peptides on MHC molecules and sequenced by MS spectrometry. Therefore, immunoprecipitation should be proven to be workable.


(III) Result
First, we wanted to test what kind of protein G bead is able to conjugate our MHCII antibody. As the result shown in figure 2, MHCII antibody was detectable when incubate antibody with protein G Sepharose bead. Whereas the protein G magnetic bead can not bind to MHCII antibody.

Next, we planned to measure the MHCII protein level after co-culturing Leishmania with dendritic cells. The outcome will provide us an appropriate time point to incubate Leishmania and dendritic cells. Judging from figure 3, MHCII protein level was increased in 6 hours and 48 hours. We then took 6 hours as our incubation time in the following few experiments.

After making sure of the co-culturing time and bead binding ability, we started our MHCII and MHCI immunoprecipitation. In figure 4, MHCII protein was detectable in immunoprecipitated supernatant while the beads plus antibody group had no MHCII signal. This render the success of immunoprecipitating MHCII molecules. On the other hand, MHCI was unable to be pull down by immunoprecipitation since the antibody can’t bind to MHCI efficiently. Only the MHCII sample hence be further proceed to LC/MS sample.

After MHCII immunoprecipitation, the MHCII complexes were eluted by acetic acid. Figure 5 shows that the eluted fractions contain MHCII molecule which means MHCII complexes are successfully eluted. The eluted fractions were then desalted by HPLC cartridge set (RP-18 ADS) to prepare the final LC/MS samples. However, the first LC/MS outcome interprets no peptide signal. That might because of the low eluted MHCII concentration and the experiment will be further modified.


Next, we planned to measure the MHCII protein level after co-culturing Leishmania with dendritic cells. The outcome will provide us an appropriate time point to incubate Leishmania and dendritic cells. Judging from figure 3, MHCII protein level was increased in 6 hours and 48 hours. We then took 6 hours as our incubation time in the following few experiments.

After making sure of the co-culturing time and bead binding ability, we started our MHCII and MHCI immunoprecipitation. In figure 4, MHCII protein was detectable in immunoprecipitated supernatant while the beads plus antibody group had no MHCII signal. This render the success of immunoprecipitating MHCII molecules. On the other hand, MHCI was unable to be pull down by immunoprecipitation since the antibody can’t bind to MHCI efficiently. Only the MHCII sample hence be further proceed to LC/MS sample.

After MHCII immunoprecipitation, the MHCII complexes were eluted by acetic acid. Figure 5 shows that the eluted fractions contain MHCII molecule which means MHCII complexes are successfully eluted. The eluted fractions were then desalted by HPLC cartridge set (RP-18 ADS) to prepare the final LC/MS samples. However, the first LC/MS outcome interprets no peptide signal. That might because of the low eluted MHCII concentration and the experiment will be further modified.

References:
1. Finkelman, F.D., et al., IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol, 1988. 140(4): p. 1022-7.
2. de Waal Malefyt, R., et al., Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med, 1991. 174(4): p. 915-24.
3. Petrovsky, N. and J.C. Aguilar, Vaccine adjuvants: Current state and future trends. Immunol Cell Biol, 2004. 82(5): p. 488-496.
1. Finkelman, F.D., et al., IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol, 1988. 140(4): p. 1022-7.
2. de Waal Malefyt, R., et al., Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med, 1991. 174(4): p. 915-24.
3. Petrovsky, N. and J.C. Aguilar, Vaccine adjuvants: Current state and future trends. Immunol Cell Biol, 2004. 82(5): p. 488-496.



