Building on the work of iGEM Uppsala
During our project, we aimed to characterise a previous BioBrick part in the lactic acid bacteria we were working with, Streptococcus thermophilus. As we aimed to improve the understanding of this organism as part of the project, we decided to focus on reporter constructs in order to test whether these were viable in S. thermophilus. We looked into the work of the Uppsala teams between 2011 and 2013, who BioBricked various chromoproteins originating from sea anenomes and corals and expressed them in E. coli. We selected a single chromoprotein for our experiments: this was the blue chromoprotein amilCP. When transformed into E. coli, amilCP produces a vibrant dark blue pigment within 24 hours of incubation. We wanted to test whether amilCP was a useful reporter construct in our lactic acid bacteria.
Experimental Design
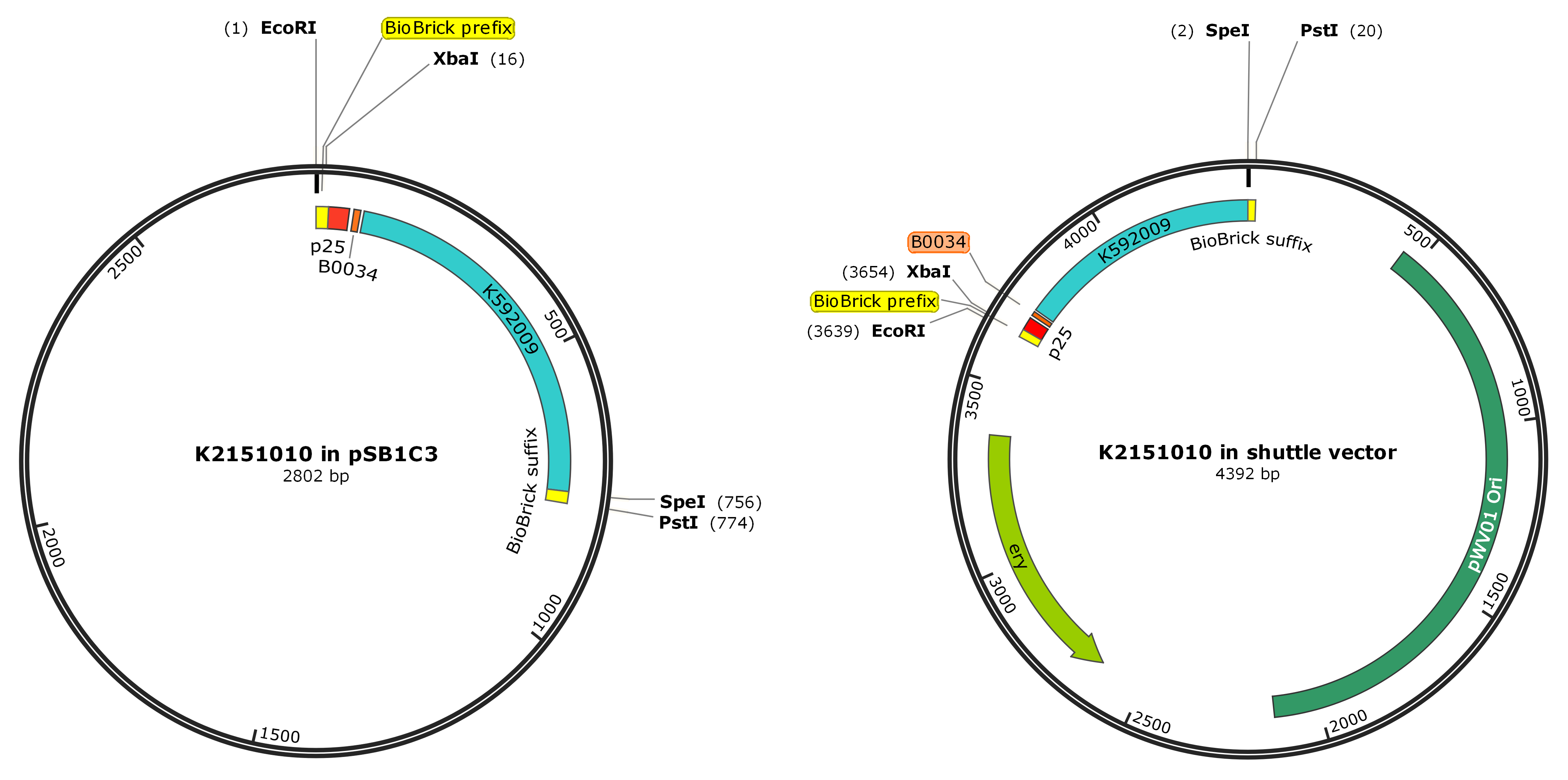
We ligated the BioBrick [http://parts.igem.org/Part:BBa_K592025 BBa_K592025] (strong RBS [http://partsregistry.org/Part:BBa_B0034 B0034] and blue chromoprotein [http://partsregistry.org/Part:BBa_K592009 amilCP]) to a native S. thermophilus promoter, P25. We then inserted the BioBrick construct into pSB1C3 and transformed it into TOP10 E. coli in order to check that the gene functioned as expected. Once this was confirmed, the construct was then inserted into the shuttle vector pMG36ET for transforming both TOP10 E. coli and S. thermophilus. This plasmid also contained an erythromycin resistance cassette in order to select for transformant colonies.
Observations and Inferences
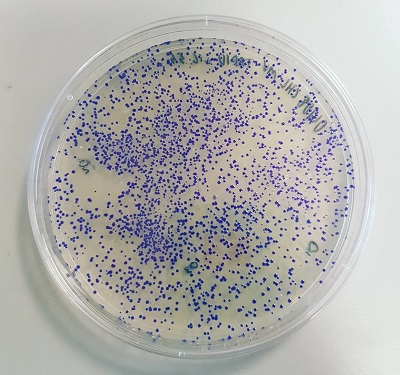
As previously described on the main page for BBa_K592025, a vibrant blue colour was produced by transformant E. coli colonies with the BioBrick construct in pSB1C3 within 24 hours (Figure 2).
Figure 2: K592025 BioBrick ligated to p25 in pSB1C3 and transformed into E. coli. A vibrant blue colour can be seen in transformant colonies.
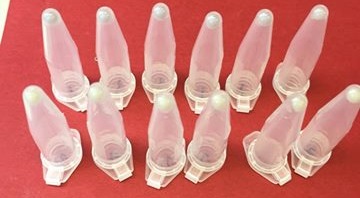
Due to the low copy number of pMG36ET, the blue colour of amilCP was less vibrant in E. coli transformed with the construct in pMG36ET than in bacteria transformed with pSB1C3. The BioBrick could still be used as a reporter in E. coli with this plasmid, but it was not as obvious a colour change as in the higher copy number plasmid, pSB1C3. When transforming the BioBrick construct into S. thermophilus, the resultant transformants (selected for with erythromycin) did not produce any blue chromoprotein even after several days. We checked that amilCP was present in this bacteria by isolating the plasmid DNA through a miniprep, and running it on an agarose gel to verify that the size was correct. We then transformed TOP10 E. coli with this miniprep, and the transformant cells were a pale blue colour.
Figure 3: Pale blue TOP10 E. coli cells which have been transformed with an S. thermophilus miniprep containing the shuttle vector plasmid with amilCP. The bottom row of cells are control cells producing no pigment.
Despite the presence of amilCP in the transformed S. thermophilus, no pigment was produced even after several days. We have come up with three hypotheses as to why this could have occurred:
- The ribosome binding site B0034 was not suitable for S. thermophilus- therefore, translation of the gene was not able to occur and blue chromoprotein was not synthesised. This is unlikely due to the consensus sequences of ribosome binding sites for both organisms being similar, with the only nucleotide base difference between the two being energetically equivalent (ACCTCCTTT/A).
- Each of the BioBrick parts in the construct was suitable for S. thermophilus, but the cellular conditions meant that the expressed amilCP was degraded before any visible pigmentation could develop.
- The low copy number of the plasmid meant that although amilCP was expressed, it was not expressed in a large enough amount to be visible to the naked eye.
It initially appears that amilCP is not a suitable reporter for S. thermophilus, however we cannot directly conclude this from our results. We would need to carry out more experiments in order to ensure the other BioBrick parts we used were all suitable for S. thermophilus before committing to the idea that amilCP is not useful in this organism.
Parts submitted to the registry
We ligated Uppsala's BioBrick [http://parts.igem.org/Part:BBa_K592025 BBa_K592025] to the three native S.thermophilus promoters we worked with throughout the project and submitted three new BioBricks. These could be utilised by teams working with S. thermophilus in order to expand upon the results we gathered.
- [http://parts.igem.org/Part:BBa_K2151009 BBa_K2151009]: phlbA ligated to [http://parts.igem.org/Part:BBa_K592025 BBa_K592025]
- [http://parts.igem.org/Part:BBa_K2151010 BBa_K2151010]: p25 ligated to [http://parts.igem.org/Part:BBa_K592025 BBa_K592025]
- [http://parts.igem.org/Part:BBa_K2151011 BBa_K2151011] : p32 ligated to [http://parts.igem.org/Part:BBa_K592025 BBa_K592025]