Cell Culture
Notebook
2016
March
28th
1. Aliquot 500ml FBS into 50ml tubes,store at -20\'e2\'84?at the first floor.
2. Prepared 500ml F12K medium, add 10%FBS(55ml)+100X PS
3. Prepared 50ml F12K medium(FBS+PS), add 10ug/ml Blasticidin+ 3ug/ml Puromycin.
4. Subcultured 1/10 geco+opn4(1:10 well) into a new well.
30th
1. Subcultured 1/10 GECO into a new well.
2. Change the culture medium of OPN4+LC3(1:10 well)
31th
1. Change the culture medium of GECO (subcultured in 3.30)
April
8th
1. Aliquot 500ml PBS into ten 50ml tubes, store at 4\'e2\'84?in cell room.
2. Subculture 1/10 GECO in to a new well.
9th
1. Change the culture medium of GECO, GECO+OPN4, OPN4+LC3.
11th
1. Discard all cell lines.
2. Identify mycoplasma infection by PCR---------Result: negative
12th
1. Recover R-GECO(1:10&3:10,PS+Bla medium) and LC3+OPN4(1:10&3:10, PS medium).
2. The R-GECO, LC3+OPN4 are stored in the most left drawer of third layer of -80\'e2\'84?
13th
1. Recovered CHO-K1(WT), 1:10&3:10, PS medium
2. The WT CHO-K1 are store in the second layer of liquid nitrogen.
3. Prepared a new 50ml medium: F-12K, 10% FBS, PS+Puromycin(3ug/ml)
4. Changed the culture medium of R-GECO(PS+Bla), LC3+OPN4(PS+Puro)
14th
1. Change the culture medium of GECO, OPN4+LC3,CHO-K1.
14th
1. Change the culture medium of GECO, OPN4+LC3,CHO-K1.
2. Subculture 1/10 GECO(1:10) and 1/10 CHO-K1(1:10) in to a new well.
3. Study how to take photos of CHO-K1 by CCD.
18th
1. Change the culture medium of GECO, CHO-K1.
21th
1. Subculture 1/10LC3+OPN4(1:5) into a new well.
2. Change the culture medium of GECO and CHO-K1
22th
1. Change the culture medium of LC3+OPN4.
24th
1. Observe the growth of CHO-K1, GECO and LC3+OPN4.
25th
- Subculture LC3+OPN4(1:10) into a new well in the origin 24-well cell cultural plate and into 4 wells in a new 12-well cell cultural plate.
26th
1. Change the cultural medium of CHO-K1, GECO and every LC3+OPN4 well.
28th
1. Changed the cultural medium of CHO-K1, GECO and 4 LC3+OPN4 wells.
2. Subcultured LC3+OPN4 in 24-well plate, 1:10. 500ul Trypsin digested for 10min.
29th
1. Changed the culture medium of LC3+OPN4(24-well plate)
30th
1. Filtration sterilization of KCl(1mol/L),10ml,store at 4\'e2\'84? 2. Subcultured GECO into 4 wells of 12-well plate. (1:5)
May
1st
1. Subculture LC3+OPN4(1:10) into a new well.
2. Change cultural medium of GECO and CHO-K1.
2nd
- Change the cultural medium of LC3+OPN4.
- Aliquot 500ml trypsin-EDTA into nine 50ml tubes and four 15ml tubes, two 15ml tubes were stored in 4 \'e2\'84?refrigerator in cell room and others were stored in -20\'e2\'84?refrigerator in 107.
3th
1. Subcultured CHO-K1 into 3 new wells of 12-well plates for transfection. (CHO-K1 WT, CHO-K1 for GFP, CHO-K1 empty vector)
2. Thawed a new R-GECO.
3. Aliquot 45ml medium: F-12K 10%FBS+PS
4th
1. Recover a new CHO-K1 cell line into three wells(1:10, 3:10, 6:10).
2. Subculture GECO(1:8) and LC3+OPN4.
3. Aliquot 50ml medium: F-12K+10%FBS+PS.
5th
1. Change the cultural medium of LC3+OPN4 and GECO1/8 & 6/10
6th
- Subculture CHO-K1(1:10 in 24-well plate) into 3 wells(1:12) in 6-well plate.
- Trypsin test\'ef\'bc?
- Subculture CHO-K1(3:10 in 24-well plate) into a new well(1:10) in 24-well, using our new trypsin(0.05%)
- Subculture CHO-K1(6:10 in 24-well plate) into a new well(1:10) in 24-well, using our new trypsin(0.25%)
7th
1. Change the culture medium of every CHO-K1 well.
8th
1. Subculture GECO into 6 wells of a new 6-well plate.
2. Subculture LC3+OPN4 into a new well of 24-well plate.(1:10)
10th
1. Transfection
Using CHO-K1 cells and Lipofectamine\'c2\'ae 3000, 6 well plate, 3 wells: untransfected WT, GFP(with intron#6), empty vector PBX084
| 125μL opti-MEM + 3.75μL Lipo3000 | => | Mix Well | ||||
|---|---|---|---|---|---|---|
| => | Mix and incubate at room temperature for 5min | => | Add into cell | |||
| 125μL opti-MEM + 2500ng DNA | => | + 5μL P3000 Mix well |
GFP(with intron)#6: 222.0ng/ul 11.3ul
PBX084: 322.5ng/μL 7.7ul
11th
1. Observe every well of CHO-K1\'e3\'80\'81\'47ECO and LC3+OPN4.
2. Fluorescence test with CCD--------Result: negative
13th
1.Live cell imaging(KCl pre-test ) KCl 50mM HEPES 40Mm R-GECO
14th
1. Change the culture medium of GECO&LC3+OPN4.
2. Subculture CHO-K1 WT into 3 new well(1:5) in 6-well plate.
15th
1. Change the medium of every CHO-K1 well in 6-well plate.
18th
1. Change the culture medium of CHO-k1, GECO and LC3+OPN4.
20th
1. Change the culture medium of CHO-K1 & HELA cell.
2. Subculture GECO into 6 wells in a new 6-well plate(1:30) and a new well in the new 24-well plate(1:10).(trypsin 0.05%)
3. Subculture LC3+OPN4 into a new well in the new 24-well plate(1:10).(Trypsin 0.05%)
21th
1. Changed the culture medium of GECO and LC3+OPN4 cultured yesterday.
22th
1. Subculture CHO-K1 into 4 wells in a new 12-well plate.
23th
1. Change the culture medium of 4 CHO-k1 wells.
24th
1. Change the culture medium of 6 GECO wells.
2. Subculture GECO and LC3 into new wells (in 24-well plate).
25th
1. Subcultured a well of R-GECO into 24 well plate.
2. R-GECO live cell imaging(KCl test)
Materials:
GECO cell plate, HBSS, 1M KCl, 1M HEPES, parafilm, pipette, tips.
Procedure
1.Initiate live cell imaging system.
2.Discard culture medium, wash with PBS three times, add 3ml HBSS into each well, wrap the plate with parafilm and bring it to R.107
3.Place the plate upon the lens, find a field with some single cells, shoot in bright field first.
4.Change to green light, set parameters, interval 200ms, total 3000 photos.
5.Turn off the light in the room, start shooting.
6.Add 1M KCl into the well during 600th shooting.
| Group | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| KCl concentration | 12.5mM | 25mM | 50mM | 100mM | 200mM |
| 1xKCl volume | 38uL | 77uL | 158uL | 333uL | 750uL |
27th
- Transfection
- Using CHO-K1 cells and Lipofectamine\'c2\'ae 3000, 12 well plate, 4 wells: untransfected WT, GFP(intron#6), PBX084(mCherry), PBX083(GFP)
| 70μL opti-MEM + 2.1μL Lipo3000 | => | Mix Well | ||||
|---|---|---|---|---|---|---|
| => | Mix and incubate at room temperature for 5min | => | Add into cell | |||
| 70μL opti-MEM + 1400ng DNA | => | + 2.8μL P3000 Mix well |
GFP(with intron)#6: 222.0ng/ul 6.3ul
PBX084: 322.5ng/ul 4.3ul
PBX083: 159.5ng/ul 8.7ul
28th
1. Observe the growth of CHO-K1, GECO and Hela cells.
2. Observe the living condition of CHO-k1 after transfection.
30th
1.Observe the fluorescence of four CHO-K1 wells with CCD.
Results: WT& GFP& empty vector(pbx084): No green or red fluorescence;
positive control(pbx083) : several cell have green fluorescence
2. Subculture CHO-k1 WT into a new well (1:10) in 24-well plate;
3. Subculture GECO into 8 new wells (1:10) in 24-well plate.
31th
1. Change the culture medium of every GECO and CHO-K1 well.
2016.6.2 22:30-23:00 LLX
1. Change the culture medium of CHO-K1, every GECO well.
June
4th
1. Observation only.
9th
1. Freeze 5 tubes of HELA cell into -80\'e2\'84?refrigerator.
14th
1. Change the culture medium of CHO-k1 & GECO.
28th
1. Thawed CHO-K1 WT cells from liquid nitrogen.
28th
1. Change the culture medium of three wells of CHO-K1.
30th
1. Subculture CHO-K1 in No.1 well into 6 wells(1:6);
2. Subculture CHO-K1 in No.2 well into 2 wells(1:5);
3. Subculture CHO-K1 in No.3 well into one well(1:10);
3th
1. Change the culture medium of every CHO-K1 well.
July
1st
1. Observer every well.
2nd
1. Subculture one well (1:6) of CHO-k1 into six new wells(1:6);
2. Subculture one well (1:6) of CHO-k1 into two new wells(1:10);
3th
Change the culture medium of every well.
1. Transfect CHO-K1 as follow:
| PBX90 Piggybac | 0.5ug | 0.83ul | 604 ng/uL |
| piezo | 0.4ug | 0.68uL | |
| Geco | 0.2ug | 0.08uL | dilute GECO first, otherwise, 0.08 is too small to pipette |
| PBX90 Piggybac | 0.5ug | 0.83ul | 604 ng/uL |
| Geco | 0.2ug | 0.08uL | |
| PBX083 | 1.2ug | 0.73ul | 1641 ng/uL |
| 50μL opti-MEM + 3μL Lipo3000 | => | Mix Well | ||||
|---|---|---|---|---|---|---|
| => | Mix and incubate at room temperature for 5min | => | Add into cell | |||
| 50μL opti-MEM + DNA + 2.8μL P3000 | => | Mix well |
4th
1. Observe cell in every well
4th
1. Subculture PBX083, GECO, GECO+PIEZO to three 10cm dishes.
5th
2. Observe cell in every well
5th
1. Observe PBX083 under fluorescent microscope.
6th
1. Observe cell in every well.
2. Change the culture medium of every well (Add Bla(10\'ce\'bcg/ml) into GECO and Bla(10\'ce\'bcg/ml)+Puro(3\'ce\'bcg/ml) into GECO+Piezo)
7th
1. Observe cell in every well.
8th
1. Subculture CHO-k1 WT, GECO, GECO+Piezo.
9th
1. Observe cell in every well and change the medium of every well.
10th
1. Subculture CHO-k1 WT, GECO, GECO+Piezo.
11th
1. Observe cell in every well and change the medium of every well.
12th
1. Subculture CHO-k1 WT, GECO, GECO+Piezo into 6-well plate.
13th
1. Observe cell in every well and change the medium of every well.
14th
1. Subculture CHO-k1 WT, GECO, GECO+Piezo.
15th
1. Subculture GECO into 4 new wells (no bla, bla(10ug/ml), bla(15), bla(20)).
2. Change the cultural medium of CHO-k1 WT.
3. Subculture GECO+Piezo (1:8) into a new well.
16th
1. Observe cell in every well and change the medium of every well.
17th
1. Observe cell in every well.
18th
1. Subculture CHO-k1 WT into 3 new well, and add bla and puro into one well respectively to teat antibiotics.
2. Subculture GECO+Piezo into 2 new wells, and one well will be use to observe fluorescence.
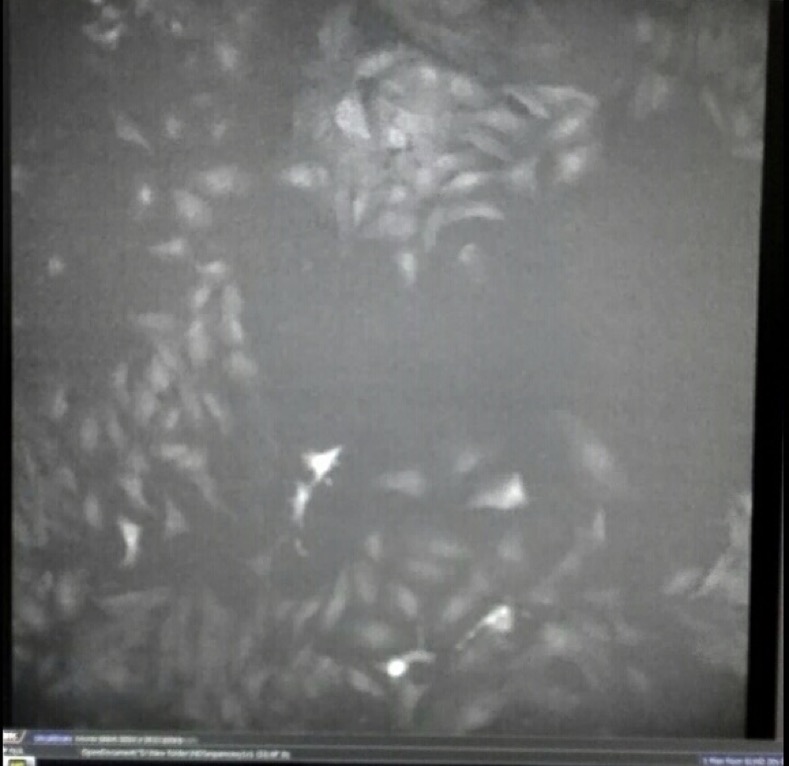
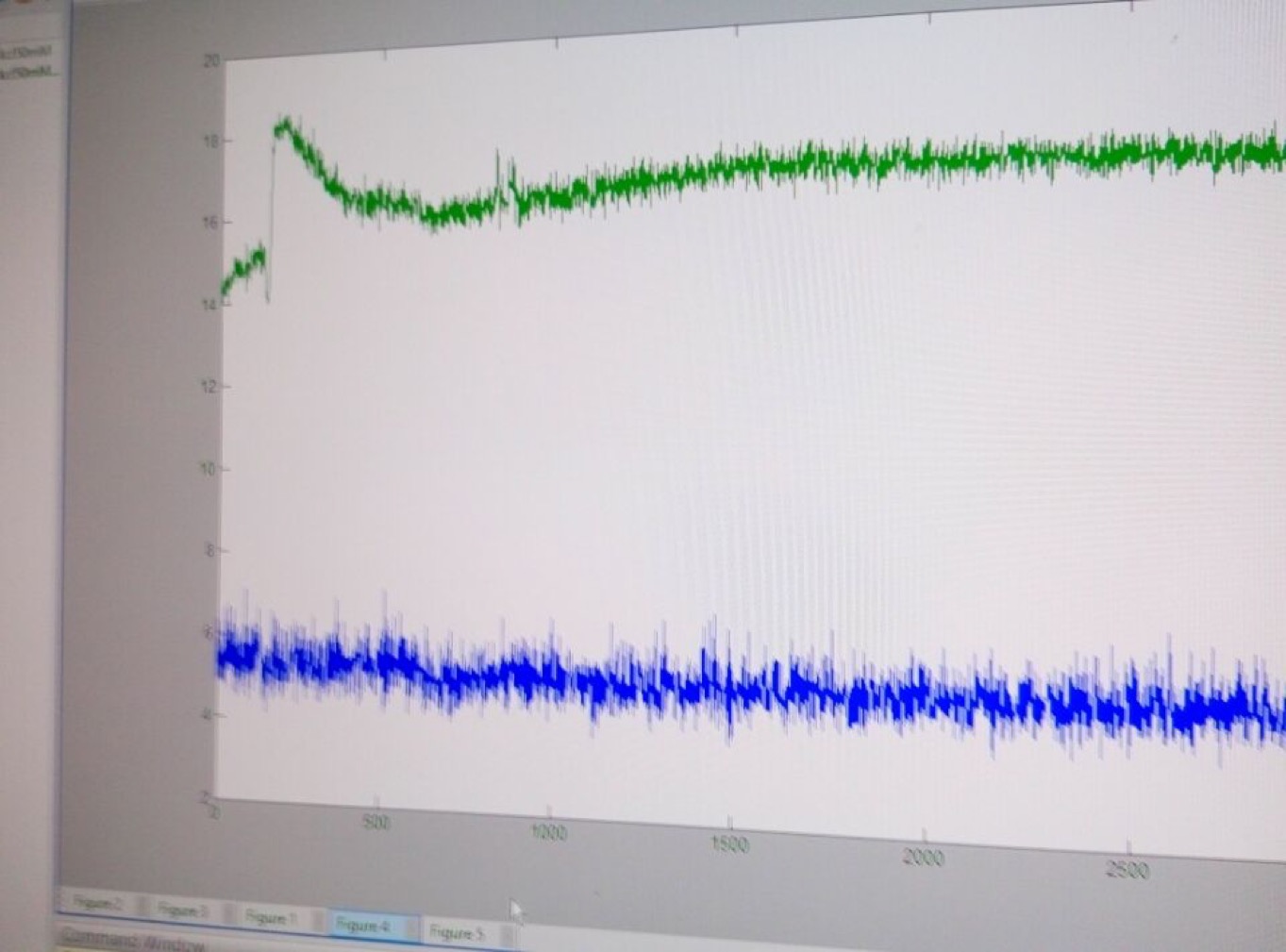
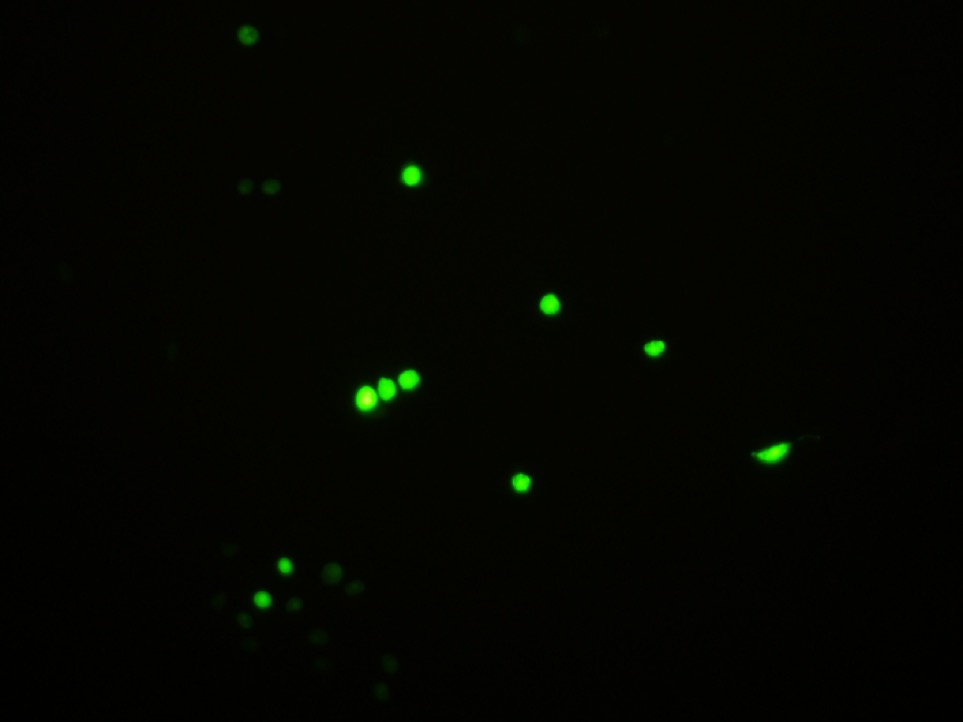
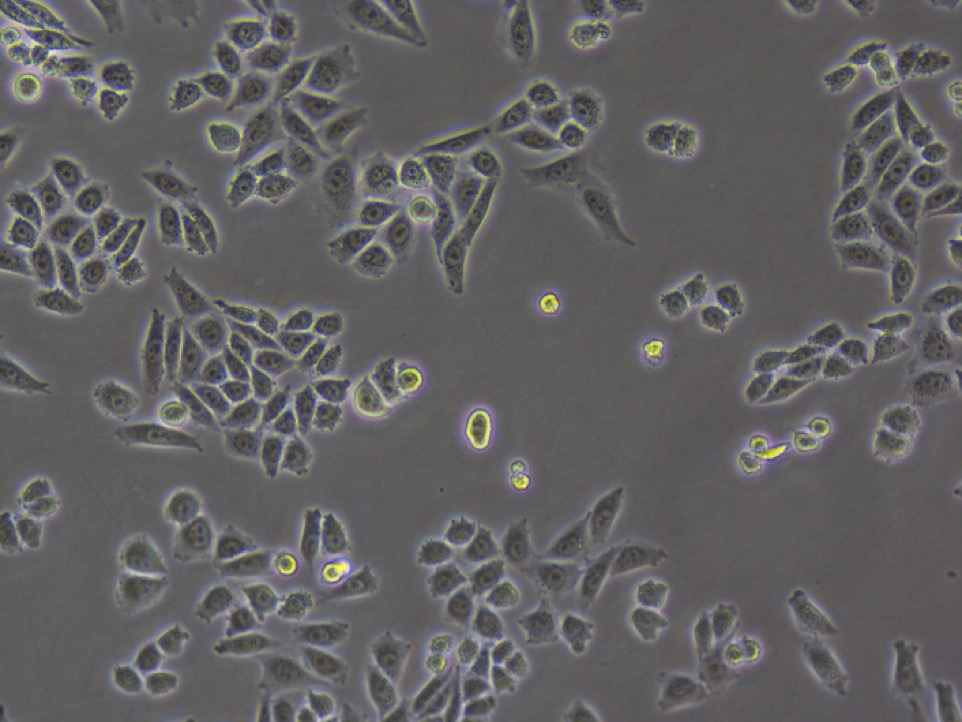
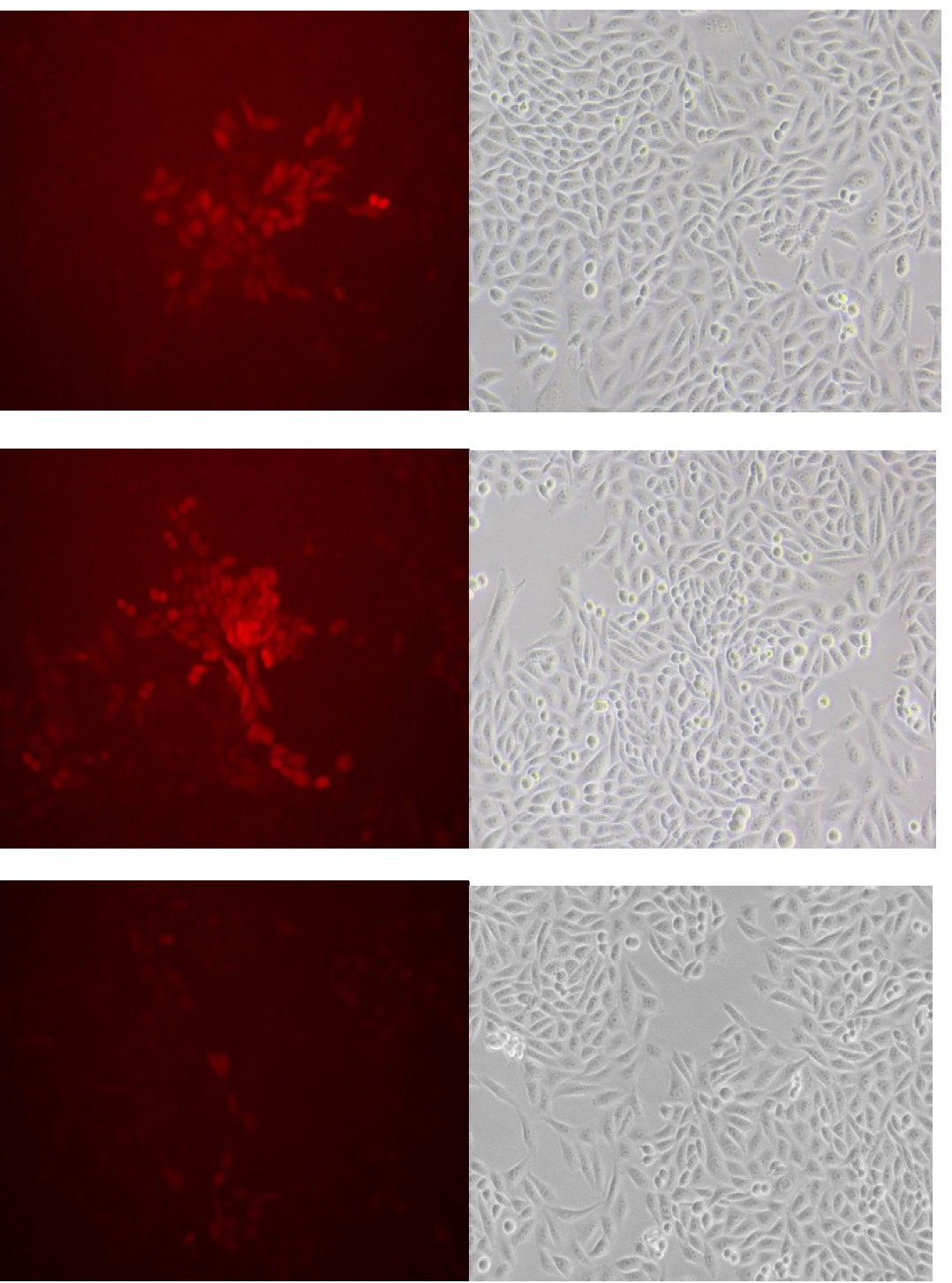
3. Observe fluorescence of GECO.
Fig30. Fluorescence of GECO (left) and bright field (right)
21th
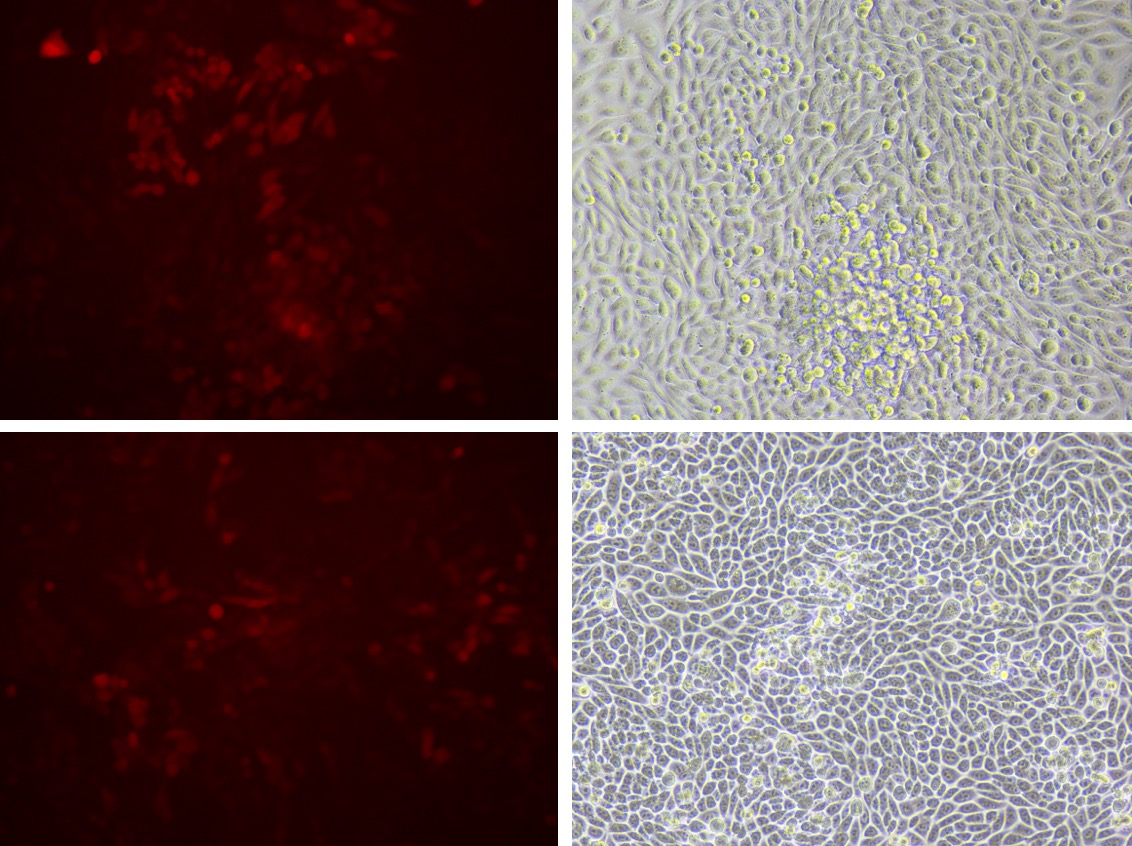
1. Observe fluorescence of GECO+Piezo.
August
8th
1. Mycoplasma test------Result: negative
12th - 16th
1. Antibiotics test
(1). Subculture CHO-k1 WT into 6 new wells, and add neo into three wells (concentration: 300ng/ml, 600ng/ml, 900ng/ml, respectively), and add zeo into another three wells (concentration: 200ng/ml, 400ng/ml, 600ng/ml, respectively)
(2). Effective concentration: neo: 600ng/ml, zeo: 400ng/ml.
July 19th - August 24 th
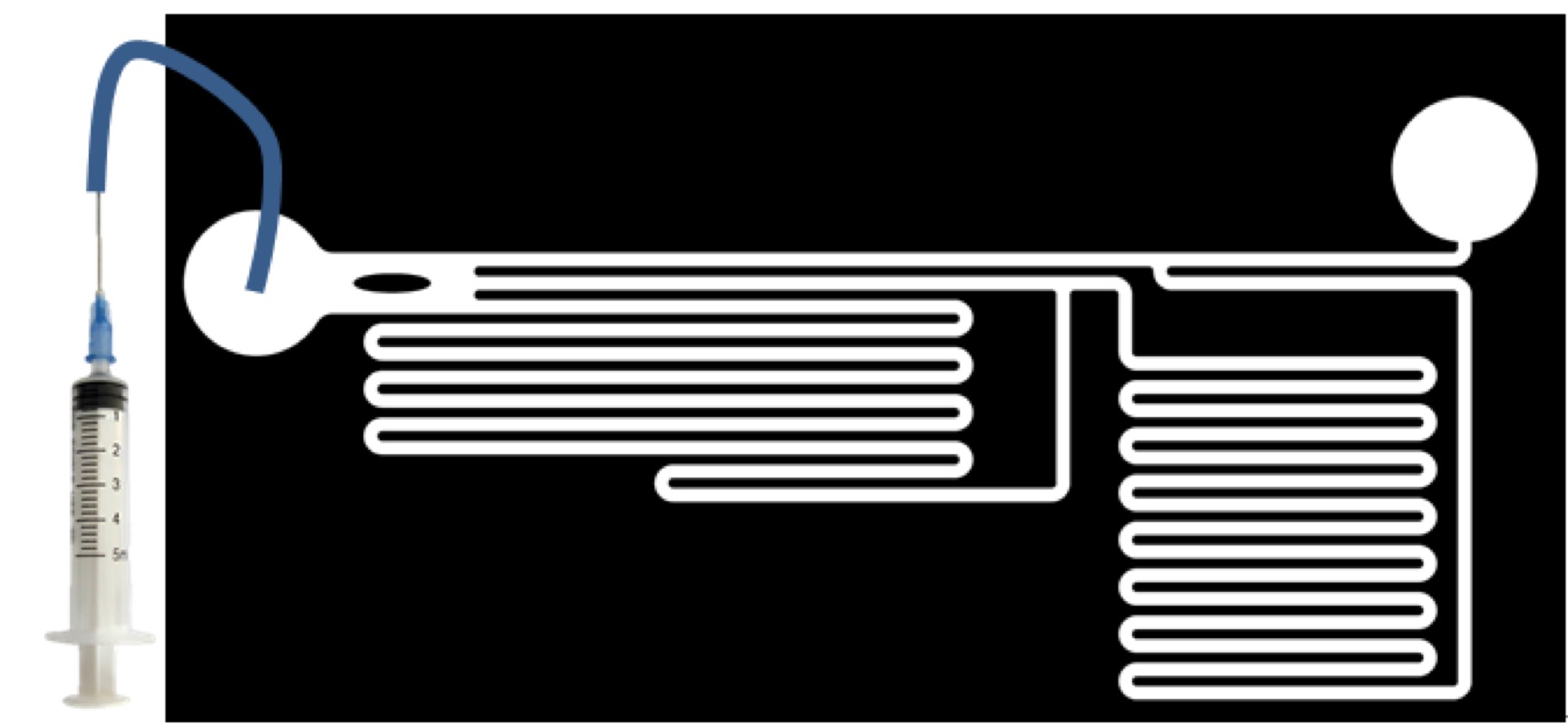
1. Cell culture in miceofluidics chips
(1). Dip chips in 70% ethanol for one day.
(2). Wash the chips with ddH2O.
(3). Dip the chips in 100% ethanol for 5 minutes.
(4). Dry the chips by air.
(5). Put the chips into a dish and add 0.5% gelatin.
(6). Treat the chips for 30 minutes in 37\'e2\'84?
(7). Discard gelatin and add culture medium into the chips.
(8). Inject cells into chips and put them in incubator.
2. Microfluidics teat
Inject culture medium in different speed(50ul/min, 100ul/min, 200ul/min, 500ul/min, 1000ul/min, 2000ul/min) into chips and observe fluorescence with live cell image system.
25th
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO.
2. Observe the reaction of cell line of GECO+PIEZO to different shear force by microfluidics, as well as we observe the cell line of GECO in the same condition as negative control (Set the speed of liquid as 50ul/min, 100ul/min, 200ul/min, 500ul/min, 1000ul/min, 2000ul/min 0f 5ml syringe which connects to the chip of microfluidics)
27 th
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO;
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate;
4. Transfer some cell (GECO/GECO+PIEZO)into two chips while we find that these new chips are easy to form bubbles.
5. Observe the growth of CHO-K1, GECO and GECO+PIEZO;
6. Transfection
3 wells of 6-well plate.
Dilute GECO first, 2382ng/ul, 238.2ng/ul(10x)
| PBX90Piggybac | 0.5ug | 0.83ul | 604ng/ul |
| GECO | 0.4ug | 1.68ul | 283.2ng/ul |
| NFAT(XJS_6) | 0.4ug | 1.41ul | 283.1ng/ul |
| 097-Neo | 0.3ug | 0.86ul | 347.9ng/ul |
| PBX90Piggybac | 0.5ug | 0.83ul | 604ng/ul |
| GECO | 0.4ug | 1.68ul | 283.2ng/ul |
| NFAT(XJS_6) | 0.4ug | 1.41ul | 283.1ng/ul |
| PBX083 | 1.2ug | 0.73ul | 1641ng/ul |
| 125μL opti-MEM + 15μL Lipo3000 | => | Mix Well | ||||
|---|---|---|---|---|---|---|
| => | Mix and incubate at room temperature for 5min | => | Add into cell | |||
| 1125μL opti-MEM + DNA + P3000(2ul/ng) | => | Mix well |
29th
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO.
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate;
4. Screen the transfected cell with antibiotics.(GECO-bla 10ug/ml; NFAT-Zeo 400ug/ml; NEOLOXP-Neo 600ug/ml)
5. We tried to repeat the experiments of microfluidics as we should investigate a best protocol, as well as, the result was influenced by temperature, quality of chips, living condition of cells and so on. This time, we found that it was hard to transfer cells into channel of different speed in chips evenly.
6. Freeze 10 tubes of CHO-K1 (transfected with GECO) cell and 10 tubes of CHO-K1(transfected with GECO&PIEZO) cell into liquid-nitrogen.
31th
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO.
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate;
4. Observe the chips made yesterday, the attachment of cells was great.
31th
1. Transfection
2 wells of 6-well plate.
| PBX90Piggybac | 0.6ug | 0.99ul | 604ng/ul |
| GECO | 0.4ug | 1.68ul | 283.2ng/ul |
| NFAT(XJS_6) | 0.4ug | 1.41ul | 283.1ng/ul |
| 097-Neo | 0.3ug | 0.86ul | 347.9ng/ul |
| TRPC5 | 0.4ug | 1.24ul | 322ng/ul |
| PBX083 | 1.2ug | 0.73ul | 1641 ng/ul |
| 125μL opti-MEM + 15μL Lipo3000(2.5X) | => | Mix Well | ||||
|---|---|---|---|---|---|---|
| => | Mix and incubate at room temperature for 5min | => | Add into cell | |||
| 125μL opti-MEM + DNA + P3000(2ul/ug) | => | Mix well |
September
1st
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO.
2. There was something wrong in NFAT plasmid, so we transfected cell again.
3. Microfluidics test
5th
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO.
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate;
4. Screen transfected cell(9.2) with antibiotics.(GECO-bla 10ug/ml; NFAT-Zeo 400ug/ml; NEOLOXP-Neo 600ug/ml)
5. Transfer cell of GECO/GECO+PIEZO into chips.
9th - 16st
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO every day.
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate every two days;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate every two days;
4. Use FACS to get the single clone of cell of GECO+NFAT and GECO+NFAT+neoLOXP into there 96-well plate.
6. Transfer cell of GECO/GECO+PIEZO into chips every day and do microfluidics test the next day.
17th
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO.
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate;
4. Subculture 14 single clone cell of GECO+NFAT+neoLOXP and 6 single clone cell of GECO+NFAT into 24-well plate a week after FACS.
19th
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO.
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate;
4. Subculture 14 single clone cell of GECO+NFAT+neoLOXP and 6 single clone cell of GECO+NFAT and resuspend the cell with PBS to extract genomic DNA .
5. Transfect cell of GECO+NFAT+neoLOXP with TRPC5 (linear backbone and annular plasmid) for pre-experiment of sound visualization experiment by cre enzyme and lipo3000.
20th
1. Observe cell and subculture.
2. Screen transfected cell (9.19) with antibiotics.(TRPC5-puro 3ug/ml)
21th
1. Observe cell and subculture.
2. Microfluidics test.
22th
1. Observe cell and subculture.
2. As the result of qPCR was not good, we sub cultured all wells in 96-well plate into 24-well plate.
3. Transfer cell into chips of microfluidics.
September 23th -October 1st
1. Observe the growth of CHO-K1, GECO and GECO+PIEZO every day.
2. Subculture CHO-k1 WT into a new well (1:10) in 6-well plate every two days;
3. Subculture GECO and GECO+PIEZO into a new wells (1:10) in 6-well plate every two days;
4. Subculture single clone of GECO+NFAT and GECO+NFAT+neoLOXP into a new wells (1:10) in 24-well plate every two days;
5. Transfer cell of GECO/GECO+PIEZO into chips every day and do microfluidics test the next day.
September 29th -October 1st
Something wrong with the chips so we fault to transfer cell into chips.
October
2th - th
1. Observe cell and subculture.
2. Recovery GECO and GECO+PIEZO in a new lab.
6th
1. Observe cell and subculture.
2. Transfer GECO/GEO+PIEZO (new cell) into new chips of microfluidics. 3.
7th
1. Observe cell and subculture (both old cell and new cell).
2. Transfection (new cell)
Two wells of 6-well plate CHO-k1 WT
Double promotors plasmid: 2ug 3.5ul
PBX083: 2ug 1.2ul 1641ng/ul
| 125μL opti-MEM + 3.75μL Lipo3000(2.5X) | => | Mix Well | ||||
|---|---|---|---|---|---|---|
| => | Mix and incubate at room temperature for 5min | => | Add into cell | |||
| 125μL opti-MEM + DNA + P3000(2ul/ug) | => | Mix well |
Two wells of 6-well plate GECO
| PBX090 | 1ug | 1.67ul | 604.8ng/ul |
| NFAT | 1ug | 3.5ul | 283ng/ul |
| PBX090 | 1ug | 1.67ul | 604.8ng/ul |
| NFAT | 1ug | 3.5ul | |
| Loxp | 0.5ug | 3.3ul | 294.2ng/ul |
One well of 6-well plate GECO+Piezo
| PBX090 | 1ug | 1.67ul | 604.8ng/ul |
| NFAT | 1ug | 3.5ul |
8th
1. Observe cell and subculture (both old cell and new cell).
2. Microfluidics test
3. Buzzers cytotoxic test. Put buzzers into the 6cm well just above the cells.