Design
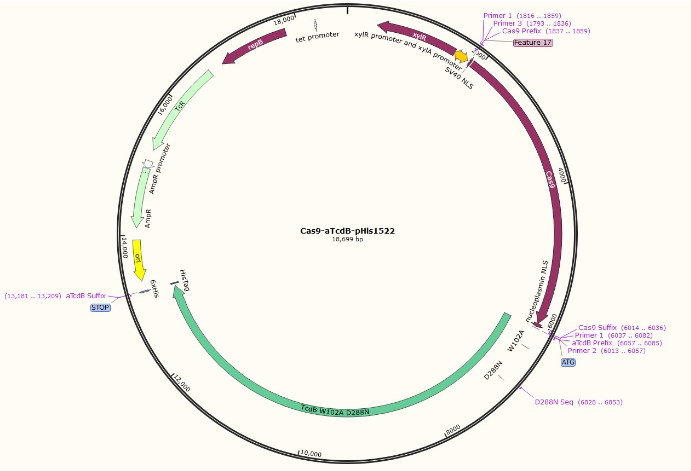
The idea behind this project is to expand upon the functions of the aTcdB delivery system by using it to deliver CRISPR/Cas9 into the cell. The toxin used is an atoxic C. difficile B (atcdB), more specifically the W102A D288N line, which is glucosyltransferase deficient. Glucosyltransferase causes apoptosis in cells, but the atoxic toxin B does not have any detrimental health effects.
The Cas9 gene from S. pyogenes is used, but also contains an N-terminal SV40 NLS tag and a C-terminal nucleoplasmin NLS tag, both of which direct the protein to the nucleus. The construct is attached to the aTcdB via a glycine-serine linker, and ends with a 6xHis tag in order to aid in the purification process. It is assembled in the pHis1522 plasmid and was transformed in DH5a E. coli. Since E. coli cannot reliably translate such large proteins, the plasmid will ultimately be transformed into Bacillus megaterium which is capable of synthesizing massive proteins. The Cas9-aTcdB protein will be expressed with a xylose inducible promoter. The C-terminal His tag on the construct will be able to bind to a nickel column during purification. The gRNA will be transcribed in vitro, and incubated with the Cas9-aTcdB.
An additional construct was designed, one which is meant to deliver NanoLuc, a highly luminescent protein, into the cell. The design is similar to the Cas9-aTcdB proetin, with NanoLuc being connected to aTcdB via a glycine/serine linker, and is tagged with a 6xHis tag. The protein is also designed in the pHIS1522 vector under the xylose inducible promoter to serve as additional proof that aTcdB can be used as an efficient protein delivery system. NanoLuc-pHis1522 is a control plasmid designed with just the NanoLuc gene and the pHIS vector, as a control to test expression under the xylose inducible promoter.




