| Line 126: | Line 126: | ||
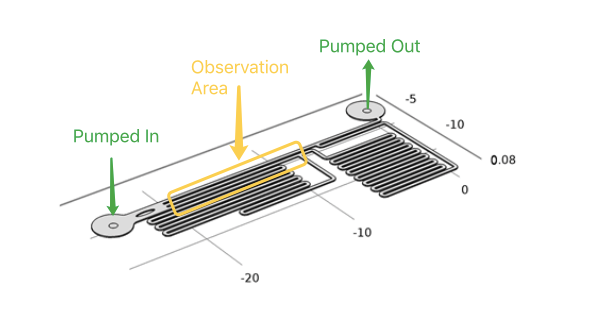
In our project, to diminish the misgiving because of transfection and quantitively analyze the following experimental result, we have to test if different shear stress will result in different cell response . With a pump applied, accurate flow rate controlling can be achieved in microfluidic chips. After calculation and simulation of fluid dynamics, relationship between the shear force on the cell membrane and fluorescence intensity of the cell can be gotten. | In our project, to diminish the misgiving because of transfection and quantitively analyze the following experimental result, we have to test if different shear stress will result in different cell response . With a pump applied, accurate flow rate controlling can be achieved in microfluidic chips. After calculation and simulation of fluid dynamics, relationship between the shear force on the cell membrane and fluorescence intensity of the cell can be gotten. | ||
| − | As the dimensions of the microfluidic devices are reduced, physics characteristics in conventional laboratory-scale is not applied. | + | As the dimensions of the microfluidic devices are reduced, physics characteristics in conventional laboratory-scale is not applied. Usually the fluid in microfludic chips is laminar fluid flow(Reynolds number<3),which has also been proved by the COMSOL simulation ''(see modeling part)''. It leads to a drastic simplification of the complex Navier-Stokes equations describing fluid mechanics. For these reasons, dedicated microfluidic instruments have been designed to precisely control fluids inside microchannels. Each time when we applied a constant pumped inflow, cells in 3 different observation tunnel could receive corresponding small, middle, large, 3 level of force magnitude(1: 9: 81). By changing the pumped flow rate, we could measure MS channel response under a series of force with different magnitude order. |
{{SUSTech_Image | filename=T--SUSTech_Shenzhen--pasted-image-124.png|caption=Fig. 2 The design of microfluidic chip}} | {{SUSTech_Image | filename=T--SUSTech_Shenzhen--pasted-image-124.png|caption=Fig. 2 The design of microfluidic chip}} | ||
Revision as of 12:16, 16 October 2016

Hardware
Model
Contents
Introduction
We are aiming to evolve a more sensitive mechanical sensitive channel (MS channel). When MS channels are stimulated by mechanical force, a Ca2+ influx is triggered. To measure the performance of MS channel, we chose sound to produce mechanical force and GECO protein as Ca2+ indicator to reflect the channel sensitivity through its fluorescence change.
Sound part
In this part, we aim to explore the effect different frequency on cell. Frequencies from 300Hz to 1.7MHz were tried to observe whether the cell with MS channel can response differently to different frequency. If so, frequency orthogonal audiogenetic parts are hopefully to be developed. Meanwhile, the equipment was also designed to be easy to change sonic power, in order to choose the most suitable intensity being harmless to cell.
Sonic Stimulation
Signal generation
A dual channel function/arbitrary waveform generator (Feel Tech FY2303) was used to generate sound wave signals. It can generate 1uHz to 2MHz, 0v to 20v signals. An amplifier was used to amplify the wave signal output by the wave generator.Sound generator
Buzzer
Buzzer is a thin metal plate attached with a piezoelectric ceramic. When varying voltage is added to its two ends, it deforms coordinately. Whereas, its low and high frequency response is weak, and can only be used to generate sound under 15 KHz. For cell safety, we fix it on the bottom of cell dish for cell stimulation.Speaker
As the low and high frequency response of buzzer is weak, speaker (Type: rsiym) was chose for complementation. Its power can reach 3W and it was drenched in culture medium for low frequency experiment.Balanced Amateur
Buzzers and speakers both have short comes. For buzzers, sound could mainly be absorbed by cell dish bottom and the power was very weak. Speaker is more powerful though, it is cell toxic and not stable. For more, they both generate a vertical pressure to cell but not shear force. So, we promoted a third plan using balanced amateur.
Balanced amateur is very stable and small. A steel tube was fixed on it to drench in to water. Thus it can generate a shear force horizontal to cell dish, which is closer to the mechanism of human hearing. The problem is that its power is not strong enough to trigger a cell response.
Lab Circuit
The following is a brief circuit of sound generator circuit.
ultra sound stimulation
Seeing cell response increased with stimulating frequency, and hearable sound can hardly trigger strong cell response. Ultrasound was come up with. There were 4 kinds of ultrasound devices constructed.
Horn-shaped ultrasound generator
We first constructed this device using circuits from ultrasound cleaner. However it was easy to destroy the circuit due to too small load.
Piezoelectric Energy Transducer.
A new ultrasound device was designed seeing the horn-shaped device is too powerful and unstable. Piezoelectric energy transducer was chose for a much slighter stimulation.
Ultrasound signal generator:
KINGCHIP SD02-JSQ-V2.4 circuit, 5V DC input. 1.7MHz output.
Power control:
The signal output from KINGCHIP SD02-JSQ-V2.4 circuit was input to the following conrol circuit designed by ourselves. Output power can be controled by changing resistance. Microproccsor Arduino UNO was rogrammed to control stimulation time pattern.
Energy Transducer:
1. 1MHz energy transducer used in fog generator was used to generate stable ultrasound. Cell toxity experiment show that it has little and tolerable cell toxity.
2. For quantified stimulation, a calibrated ultrasound energy transducer was used.
Data
Diameter: 8.5mm
Thinness: 22mm
For 23Vp-p 1MHz input, 1.9W/cm2 (3mm for from head)
Lab Circuit
Device I
Two 1MHz low power ultrasound stimulators are implemented for program controlled exeriment.
Device II
One 1MHz ultrasound stimulator was implemented with larger size and maximun power. Control part and electric part were seperated into two layers for safety.
1. Stimulation program loading.
2. Fix stimulator in proper position.
3. Turn on the switch and change resistance for proper ultrasound intensity.
4. Start stimulation experimrnt.
Microfluidics
Why Microfluidics
Microfluidics is a multidisciplinary field intersecting engineering, physics, chemistry, biochemistry, nanotechnology, and biotechnology, with practical applications in the design of systems in which low volumes of fluids are processed to achieve multiplexing, automation, and high-throughput screening.[1] Microfluidic instrument allows fluids to pass through different tunnels of different diameter, usually ranging from 5 to 500 μm.[2] Owning to its characteristic of small dimension. It can save reagents and also haves smaller requirement of samples. In the last decades, the basic techniques of microfluidics for the study of cells such as cell culture, cell separation, and cell lysis, have been well developed. Based on cell handling techniques, microfluidics has been widely applied in the researches of the cell biology.[3]
In our project, to diminish the misgiving because of transfection and quantitively analyze the following experimental result, we have to test if different shear stress will result in different cell response . With a pump applied, accurate flow rate controlling can be achieved in microfluidic chips. After calculation and simulation of fluid dynamics, relationship between the shear force on the cell membrane and fluorescence intensity of the cell can be gotten.
As the dimensions of the microfluidic devices are reduced, physics characteristics in conventional laboratory-scale is not applied. Usually the fluid in microfludic chips is laminar fluid flow(Reynolds number<3),which has also been proved by the COMSOL simulation (see modeling part). It leads to a drastic simplification of the complex Navier-Stokes equations describing fluid mechanics. For these reasons, dedicated microfluidic instruments have been designed to precisely control fluids inside microchannels. Each time when we applied a constant pumped inflow, cells in 3 different observation tunnel could receive corresponding small, middle, large, 3 level of force magnitude(1: 9: 81). By changing the pumped flow rate, we could measure MS channel response under a series of force with different magnitude order.
Syringe pump programmed for stimulation of accurate intensity and duration. In our experiment, fluid flow will maintain about 2 min. After the cell recovered ( indicated by reverting to normal fluorescence intensity), next round of shear force stimulation would start over.
Cell must be tightly coherent on the channel bottom, or the cell components washed away by fluid would result in larger fluid velocity that influenced our measurement results. To deal with this problem, gelatin was applied. By treating microfludics tunnel with gelatin, cell could adhere to PDMS’s surface more tightly. Once the cell had been adhered to PDMS, syringe pump was used to control the influx of medium to generate a shear force in each tunnel. The fluorescence intensity would increased within a second in the cell line when using R-GECO as output of calcium signal. Live cell imaging system captured its signal and recorded the data throughout whole experimental period, then, Matlab analysis was applied. For the cell line has longer response time employing NFAT signal pathway, the change of gene expression(green fluorescent as downstream) can be observed under live cell imaging system after 20 hours.
Microfluidic chips in our experiment were made by polydimethylsiloxane (PDMS) fixed on a slide of glass. PDMS is the material for fast prototyping microfluidic devices. PDMS chips are widely used in laboratories, especially in the academic community due to their low cost and ease of fabrication. Here are listed the main advantages of such chips:
- Oxygen and gas permeability
- Optical transparency, robustness
- Non toxicity
- Biocompatibility
- No water permeability
One of the main drawbacks of PDMS chips is its hydrophobicity. Consequently, introducing aqueous solutions into the microchannels is difficult and hydrophobic analytes can adsorb onto the PDMS surface. Fortunately, there are now PDMS surface modification methods such as gas phase processing methods and wet chemical methods (or combination of both) to avoid issues due to hydrophobicity which we also employed in our experiment. After the fabrication of microfluidics channels having been done made by polydimethylsiloxane, they are treated with oxygen plasma for hydrophilic property. After this procedure, polydimethylsiloxane could bond to glass tightly.
How to fabricate
After reading existing papers about the processing technology typically used in microfluidic chips production and endless trial experiment, we have formed our own processing protocol based on our equipment and requirement.
The microfluidic chips used in our project were all produced using this protocol.
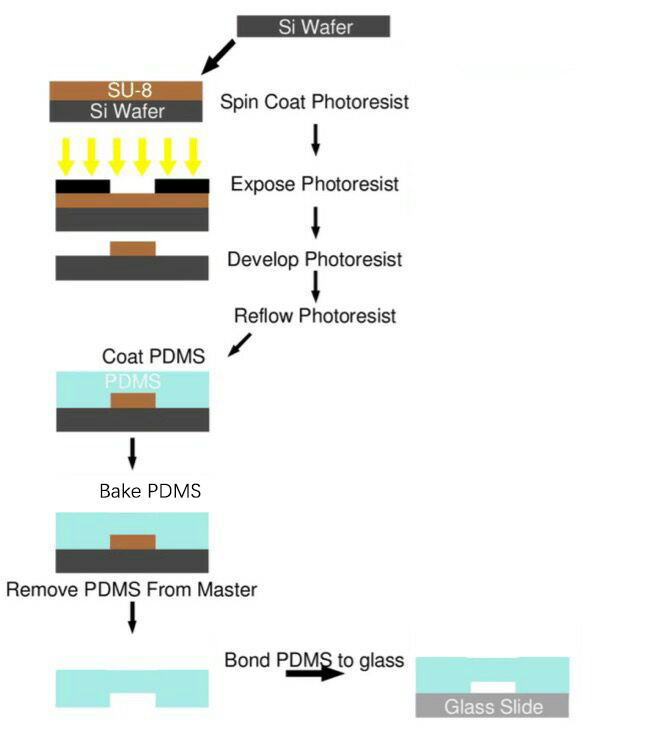
Process Overview
SU-8 Photoresist can cross-link to a network of polymer when exposed to short wavelength light. If the cross-linked network has formed, it cannot be dissolved by the SU-8 developer. So if we place a photo mask to shade the light shine on the photoresist, we can make a copy of the pattern on the photo mask.
Both PDMS and glass contains silicon element. When they were treated by oxygen plasma, unstable hydroxyl group will form on the treated surface. When they get close enough, two hydroxyl group will dehydrate and bond together covalently.
Detailed Protocol
Wash the glass mask holder by socking it in dilute ammonia water for over 1 hour, and refresh it with deionized water.
Wash the glass mask holder by socking it in dilute hydrochloric acid for over 1 hour, and refresh it with deionized water.
Wash the glass mask holder by socking it in positive photoresist remover for over 1 hour, and refresh it with deionized water.
Dry the glass mask holder in the drying oven.
Clean the mask film by wiping the two sides carefully on one direction with a clean paper socked in acetone.
Clean the mask for the second time film by wiping the two sides carefully on one direction with a clean paper socked in alcohol or isopropyl alcohol.
Fix the mask film on to the mask holder with tape.
The tape can only touch the edges of the mask film in order to prevent contamination.
Pour some SU-8 2100 photoresist in a small bottle, keep it still in the refrigerator for at least a week(two weeks recommend) to remove the bobbles inside the photoresist.
Take the SU-8 photoresist out from the refrigerator, wait until the bottle hit room temperature, and carefully pour it on to a clean silicon wafer. The bottle should be held as close to the wafer as possible to prevent bobble from generating.
Spin the wafer with photoresist on the spin coater with the following procedure.
Spin at 500 rpm for 10 seconds with acceleration time of 5 seconds.
Spin at 2000 rpm for 30 seconds with acceleration time of 5 seconds.
Spin at 500 rpm for 5 seconds with acceleration time of 5 seconds.
Stop with acceleration time of 5 seconds.
Bake the wafer on 65℃ hotplate for 5 minutes.
Bake the wafer on 95℃ hotplate for 30 minutes.
Keep the wafer still in room temperature for more than 1 hour and wait until the photoresist is dry enough.
Clean the backside of the wafer with a clean paper socked in SU-8 developer to remove excessive photoresist.
Fix the wafer to the mask aligner.
Place the mask holder on the wafer carefully.
Expose the wafer with the mask aligner with the exposure energy of 220 mJ/cm2.
Bake the wafer on 65℃ hot plate for 5 minutes.
A visible pattern identical to the photomask will be seen in the film within 5 seconds after being placed on the hotplate.
Bake the wafer on 95℃ hot plate for 12 minutes.
Keep the wafer still in room temperature for 15 minutes and wait until the photoresist is dry enough.
Pour fresh SU-8 developer into the container. Place the container on the shaking table and set the speed to 400rpm. Start the shaking table and wait for the speed to be stabilized.
Put the wafer in the container and develop for 4 minutes.
Spray and wash the developed image with fresh developer for approximately 10 seconds.
Spray and wash the developed image with isopropyl alcohol for approximately 10 seconds.
Air dry the wafer with nitrogen gun.
Bake the wafer on 150℃ hot plate for 20 minutes.
Check the developed image.
Wait for the wafer to cool down.
Check the final image of the photoresist. The final image usually has a lot of defects at the edges of the channels. And due to the low transmittance of SU-8 photoresist to the low-wavelength light, the photoresist at the top side of the channel will absorb higher energy than the photoresist at the bottom, causing a trapezoidal cross section profile.
Mix about 30g PDMS base with about 3g curing agent (at the ratio of 10:1).
Wrap the wafer with tinfoil and pour the PDMS mix on to the wafer.
Put the wafer in the vacuum dryer to remove the bobbles inside.
Bake the wafer in the 80℃ drying oven for 3 hours.
Take the wafer out and rip off the tinfoil.
Carefully remove PDMS from the wafer and cut it into pieces on a piece of clean paper.
Spray and wash the PDMS with alcohol.
Spray and wash the PDMS with deionized water.
Air dry the wafer with nitrogen gun.
Soak glass slides in 100g/L NaOH solution for 14~24 hours.
Take the glass slides out, spray and wash them with deionized water.
Spray and wash the glass slides with alcohol.
Spray and wash the glass slides with deionized water.
Air dry the wafer with nitrogen gun.
Place the clean slides on a piece of tinfoil.
Dry the glass slides in the drying oven.
Clean the glass slide in the ultra-violet ozone cleaner for 15 minutes.
Take the slides out and wrap them with another piece of tinfoil.
Punch the holes for the tube on the PDMS.
Open the plasma cleaner and place the PDMS and the slide in the meddle. A piece of tinfoil should be placed under the PDMS.
Treat them with oxygen plasma for 1.5 minutes at 300W and the oxygen flow rate of 0.6mL/min.
Stop the plasma cleaner and open the cover as soon as possible. Press the treated sides together and apply pressure in 5 seconds.