| Line 23: | Line 23: | ||
</p> | </p> | ||
</div> | </div> | ||
| + | </div> | ||
| + | <div class="contentRow"> | ||
| + | <h1> Fructose test </h1> | ||
</div> | </div> | ||
<div class="contentRow"> | <div class="contentRow"> | ||
| − | + | <div class="contentCell"> | |
| − | <div class="contentCell | + | |
<figure> | <figure> | ||
<img src="https://static.igem.org/mediawiki/2016/b/bc/Tuebingen_Fructosetest.jpeg" /> | <img src="https://static.igem.org/mediawiki/2016/b/bc/Tuebingen_Fructosetest.jpeg" /> | ||
| Line 34: | Line 36: | ||
</figure> | </figure> | ||
</div> | </div> | ||
| − | |||
<div class="contentCell"> | <div class="contentCell"> | ||
| − | <p> Hier HIe Fructose TEst </p> | + | <p> Hier HIe Fructose TEst </p> |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</div> | </div> | ||
</div> | </div> | ||
Revision as of 22:15, 19 October 2016


Results
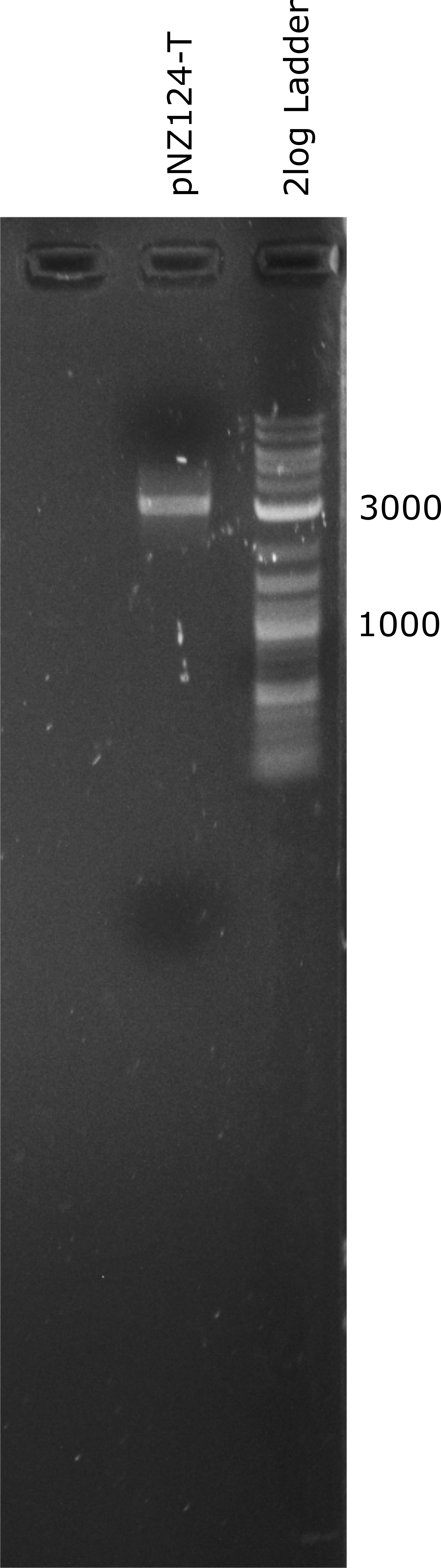
To introduce our targets for overexpression into L. johnsonii we planned to use the pNZ124 shuttle vector. We were able to ligate it from IDT part sequences and expressed it succesfully in E. coli. Before cloning the enzymes into the vector we introduced a terminator general terminator sequence right after the multiple cloning site (MCS). In Figure 1 a linearized terminator containing vector (pNZ124-T) can be seen. The expected length of the pNZ124-T is 2866 bp.
We were able to confirm some of our parts via analytical digest followed by an agarose gel electrophoresis (Figure 2). Some of those parts were additionally approved by sequencing. These parts were Phosphofructokinase and Ketohexokinase, as well as the parts PezT, the zinc fingers ZF ZFN-L, ZF K230R, ZF-C7C7 and the composite part Kill1 of our side project Clone Wars.
Fructose test

Hier HIe Fructose TEst

