| (32 intermediate revisions by 5 users not shown) | |||
| Line 6: | Line 6: | ||
{{:Team:SUSTech_Shenzhen/main-content-begin}} | {{:Team:SUSTech_Shenzhen/main-content-begin}} | ||
| − | = | + | = Overview = |
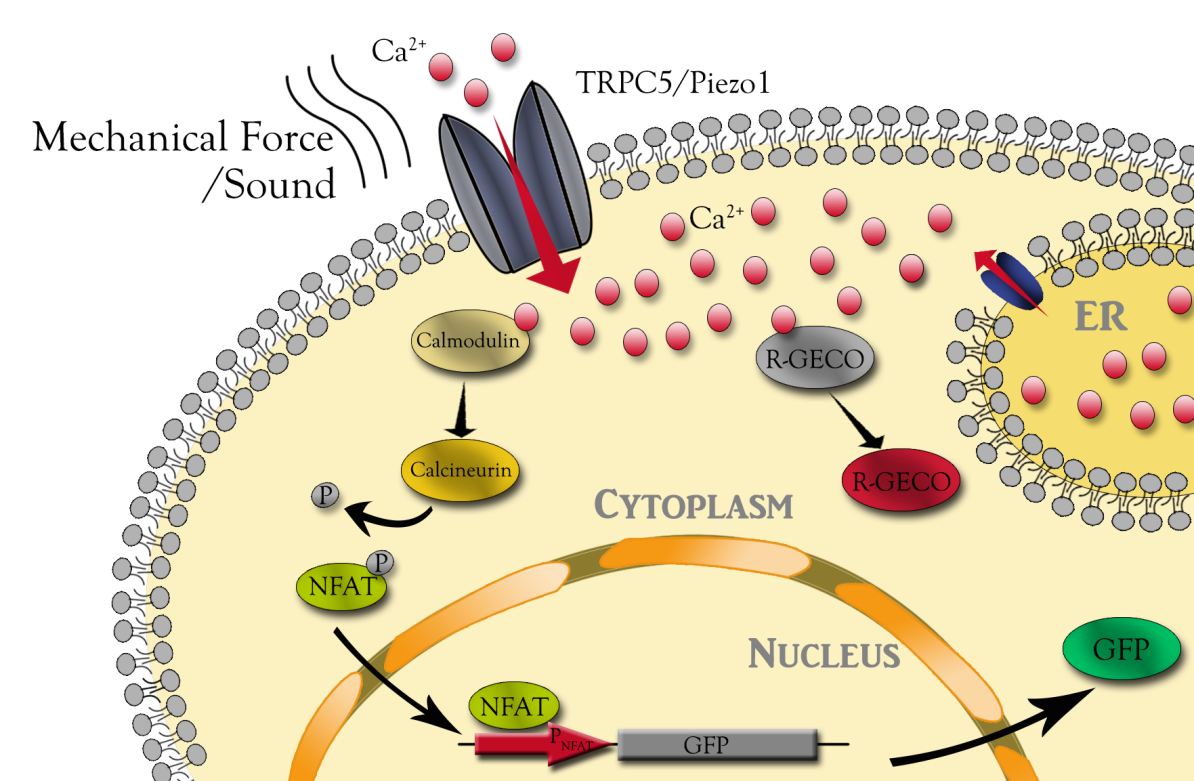
| − | + | For most cells, the molecular mechanism of sensing mechanical force starts with mechanical sensitive (MS) ion channels. The key objective of this study is to explore the possibility of using MS channels to sense sound instead. | |
| − | + | Here we adapt synthetic biology approaches to engineer non sensory neuron to detect different aspects of sound, such as frequency and intensity (Fig. 1). Mechanosensitive channels TRPC5 and Piezo1 were chosen as putative receptors of sound. We engineered downstream calcium sensor to visualize channels’ response to sound. Different channels might have different responses even exposed to the same condition. Quantitative characterization of these channels will help us to comprehend the MS channel expression cell as a whole. The downstream NFAT reporter can be used to regulation extrageneous gene expression for further cell function control, as well as for functional selection of mutant channel library. | |
| − | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--DZ1.png |width=1000px|caption=<B>Figure 1. Overview of responses to mechanical stimuli with our engineered CHO cells.</B>}} | |
| − | + | = 1. Establish indicator of channels’ response = | |
| − | + | To visualize channels’ response, we established the systematic characterization tools to analyze the response of MS channel-expression CHO cell to mechanical force. Mechanosensitive channels will induce calcium influx into cells after receiving stimulation. Cytosolic calcium increase is visualized by R-GECO, a genetically encoded calcium sensor which can emit red fluorescence upon calcium binding. Red fluorescence can be captured and analyzed by live cell imaging system. | |
| − | == | + | == Mechanosensitive channels: Piezo1 & TRPC5 == |
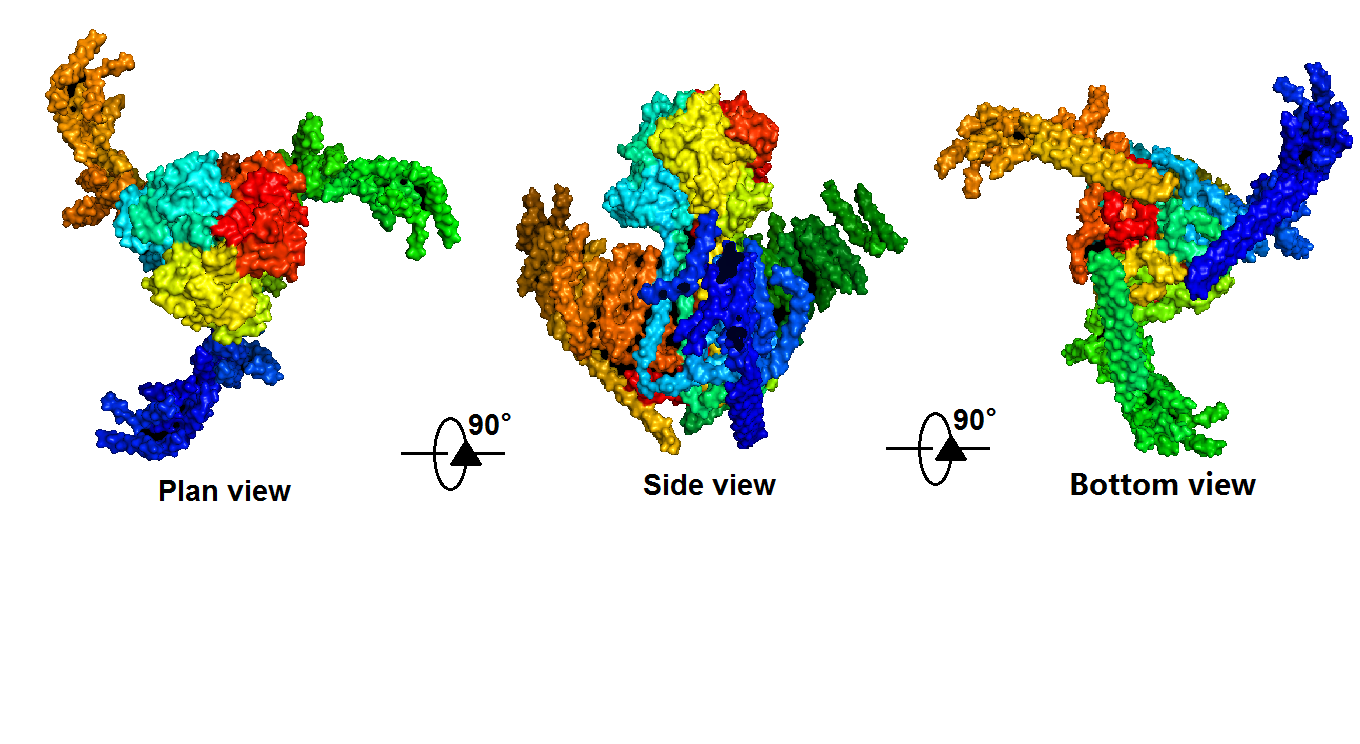
| − | + | Piezo1 protein is a functionally diverse mechanosensitive cation channel. It is expressed in lungs, bladder and skin, where mechanosensation plays an important biological role. It also plays significant role in multiple physiological processes, including sensing shear stress of blood flow for proper blood vessel development, regulating red blood cell function and controlling cell migration as well as differentiation. Despite the functional importance and high sensitivity of Piezo1 proteins, their gating mechanisms and three-dimensional (3D) structures are yet to be defined. </ref>Ge, J., et al., Architecture of the mammalian mechanosensitive Piezo1 channel. <ref> Nature, 2015. 527(7576): p. 64-9. </ref> The density map revealed that Piezo1 formed a three-blade, propeller-shaped architecture, with distinct regions resembling the typical structural components of a propeller, including three blades and a central cap. (full length, 2,547 amino acids) (Fig. 2,3). | |
| − | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--DZ2.png |width=1000px|caption=<B>Figure 2. The 3D density map of Piezo1 in a surface mode by PyMOL Viewer(from PDB database)</B>}} | |
| − | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--DZ3.png |width=1000px|caption=<B>Figure 3. A proposed model of force-induced gating of Piezo channels.<ref>Ge, J., et al., Architecture of the mammalian mechanosensitive Piezo1 channel. Nature, 2015. 527(7576): p. 64-9.</ref></B>Represent the closed and open state channels, respectively.Red dashed lines indicate the possible ionconduction pathways.Presumably, force-induced motion (red arrows) of the peripheral blade or PHs leads to conformational arrangement and | |
| + | gating of the channel.}} | ||
| − | + | Transient receptor potential (TRP) channels belong to a diverse family of cation channels that respond to a variety of signals. <ref>Flockerzi, V., An introduction on TRP channels. Handb Exp Pharmacol, 2007(179): p. 1-19.</ref><ref>Ramsey, I.S., M. Delling, and D.E. Clapham, An introduction to TRP channels. Annu Rev Physiol, 2006. 68: p. 619-47.</ref> TRPC5 is a calcium permeable cation channel predominantly express in the central nervous system (CNS). There is an impressive array of other activators of TRPC5 channel, such as nitric oxide, lysophospholipids, sphingosine-1-phosphate, reduced thioredoxin, protons, lanthanides, and calcium, among them many can cause the change of TRPC5 configuration. Moreover, TRPC5 shows constitutive activity. It is shown to be associated with membrane stretch and cold feeling. Thus, TRPC5 channel has significant potential for synergistic activation and may serve as an important focal point in calcium signaling and electrogenesis. The biological functions of TRPC5 channel are also important, ranging from neurotransmission to control of axon guidance, vascular smooth muscle cell migration and contractility. <ref>Zholos, A.V., Trpc5. Handb Exp Pharmacol, 2014. 222: p. 129-56. </ref> | |
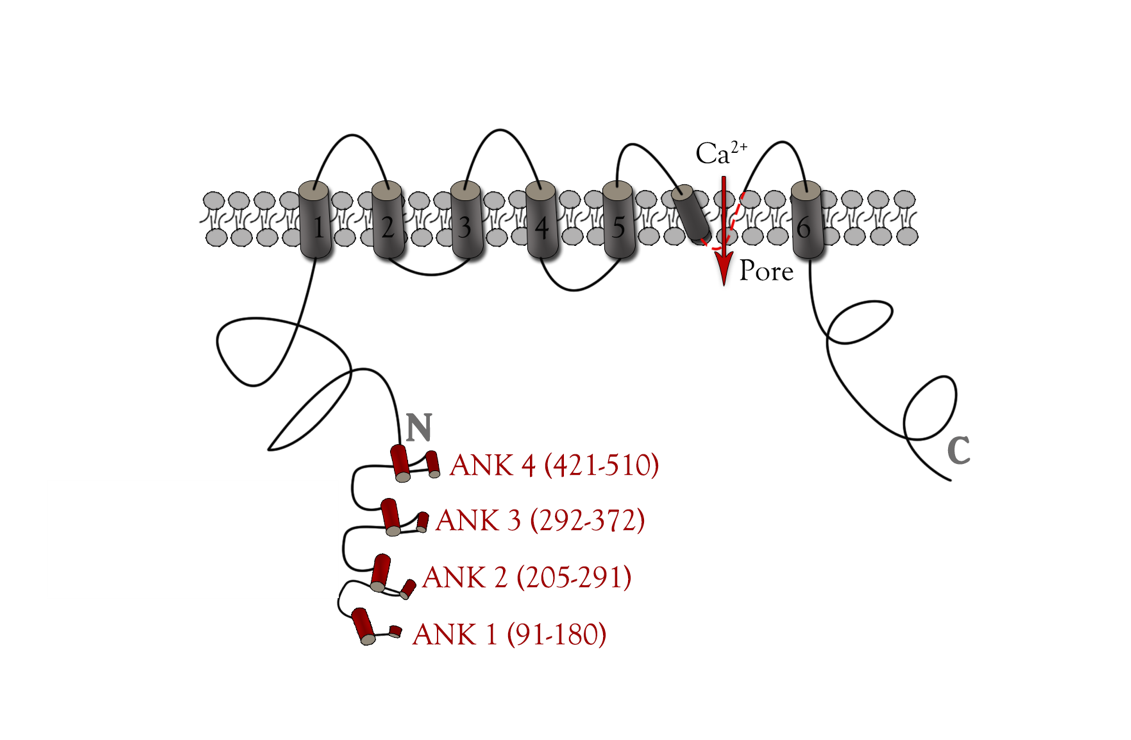
| − | + | TRPC5 shares several common features with other members of the TRP superfamily of ion channels, such as membrane topology and high selectivity to cations over anions. <ref>Owsianik G, Talavera K, Voets T et al (2006) Permeation and selectivity of TRP channels. Annu Rev Physiol 68:685–717.</ref><ref> Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647</ref> The channel core consists of six helical transmembrane (TM1–6) segments linked by extracellular and intracellular segments of variable length and flanked by the cytoplasmic N (Nt, 330 aa)- and C (Ct, 351 aa)-termini(Figure 4). A putative pore region including pore helix between TM5 and TM6 lines the channel pore when a functional channel is formed by assembling four subunits. <ref>B. Nilius and V. Flockerzi (eds.), Mammalian Transient Receptor Potential (TRP) Cation Channels, Handbook of Experimental Pharmacology 222.</ref> | |
| − | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--DZ4.png |width=1000px|caption=<B>Figure 4. Main structure of TRPC5 channel</B>}} | |
| − | + | There are several general mechanisms for mechanical activation of an ion channel. These include 1) direct channel activation by altering bilayer tension/bending/thickness, 2) indirect channel activation via mechanosensitive signaling molecules, and 3) direct channel activation by tethering to cytoskeletal elements that are exposed to mechanical forces. <ref>Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007; 428:183–207. Epub 2007/09/19. | |
| + | </ref><ref>Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007; 8(7):510–21. Epub 2007/06/23.</ref> Based on previous study exploring TRP channels gating mechanism, we know that multiple ankyrin repeats in the channel can form a helical structure, which may act as a gating spring. <ref>B. Nilius and V. Flockerzi (eds.), Mammalian Transient Receptor Potential (TRP) Cation Channels, Handbook of Experimental Pharmacology 222.</ref> Thus we chose ankyrin repeats as directed mutation region, | ||
| − | + | == Fluorescent component R-GECO (Calcium Indicator) == | |
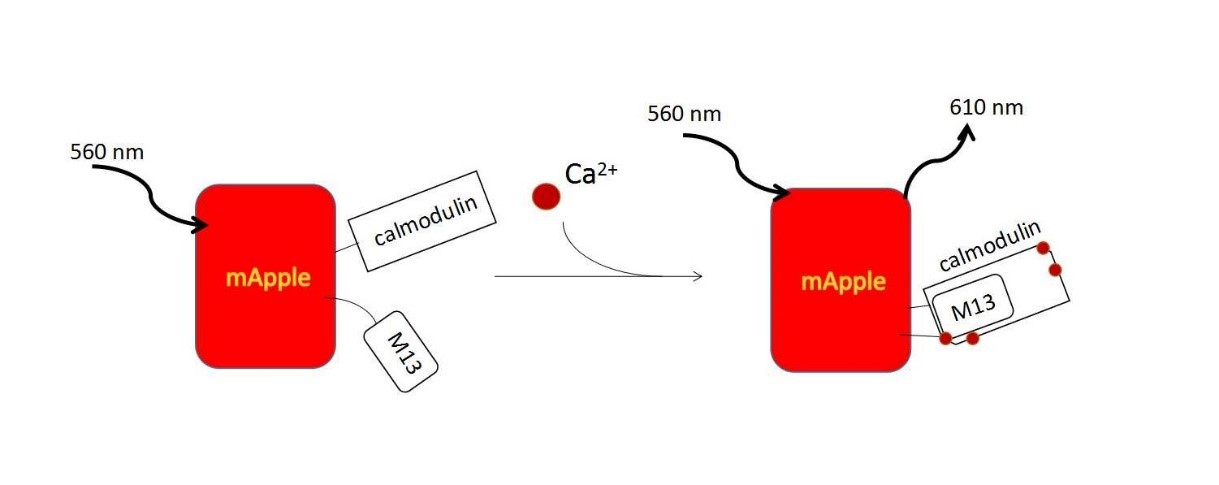
| − | + | R-GECO consists of a circularly permuted mApple red FP, which is flanked on one side by the calcium-binding protein calmodulin and on the other side by the calmodulin-binding peptide M13. In the presence of calcium, calmodulin-M13 interactions elicit conformational changes in the fluorophore environment that leads to an increase in the emitted fluorescence. (Fig. 5).<ref> Zhao, Y., et al., An expanded palette of genetically encoded Ca(2)(+) indicators. Science, 2011. 333(6051): p. 1888-91.</ref> They are useful in neuronal activity study and cell imaging for calcium indicator. We could use following methods to induce calcium influx to quantitatively examine the function of R-GECO. | |
| − | + | === Depolarization === | |
| − | + | Potassium ion is involved to make a depolarization and lead to the increases in cytosolic free calcium.<ref> Ranade, S.S., et al., Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A, 2014. 111(28): p. 10347-52.</ref> | |
| − | = | + | === Ionomycin === |
| − | < | + | Ionomycin is an ionophore produced by the bacterium Streptomyces conglobatus. It is used in research to raise the intracellular level of calcium (Ca2+) by stimulating store-regulated cation entry. <ref>Morgan, A.J. and R. Jacob, Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J, 1994. 300 ( Pt 3): p. 665-72..</ref> |
| − | </ | + | |
| − | + | ||
| − | = | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--DZ5.jpg |width=1000px|caption=<B>Figure 5. The Mechanism of Calcium Indicator</B>}} |
| − | + | = 2. Plasmid construction = | |
| − | + | {{SUSTech_Image | filename=T--SUSTech_Shenzhen--4989943B-6AD0-4AB4-931B-ADD190600E8B.png | caption=<B>Figure 6. Plasmid construction of different parts </B> ** R-GECO: R-GECO is a gift from Prof. Yue's lab. ** pBX097 backbone is provided by Prof. Huang's lab. | width=900px}} | |
| − | == | + | <html><a href="/Team:SUSTech_Shenzhen/Design/Plasmid_Construction" class="btn btn-default"><i class="ion-arrow-right-c"></i> See Details</a></html> |
| − | + | = 3. Quantitative characterization of Piezo1 and TRPC5 = | |
| − | + | Mechanical stimuli are generated by three ways: 1) Hypoosmolarity; 2) Sonic wave; 3) Microfluidics | |
| − | + | To quantitatively characterize these two channels, we employed hypoosmolarity and different magnitude of shear stress to stimulate cells. Besides, we also explored new manipulation using sound with different intensities and frequencies to test cells’ response. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
== Hypoosmolarity == | == Hypoosmolarity == | ||
| − | + | After serial dilution of medium, we used osmotic pressure to generate mechanical stress exert on cell plasma membrane to activate Piezo1 or TRPC5 channel. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Diluted medium could induce a sharply increase in fluorescence intensity, reveals that cell could sense this stress, in other words, hypoosmolarity can trigger calcium influx through Piezo1 and TRPC5 channel. | |
| − | = | + | == Sound == |
| − | + | In this part, we supposed that different frequencies and intensities would have great different impact on cells, so we employed a wide range of frequency and intensity of sound to stimulate the cell. We used home-made sound generators utilizing piezo buzzers, balanced armatures, speakers, ultrasound transducers and atomisers to activate Piezo1 and TRPC5 channel. Fluorescence intensity increase could be observed after intracellular calcium concentration increased. There is little literature report of quantitive research on channels employing sound as stimulus, we wanted to quantify our experiment, simulation and calculation of how much energy we should apply on cell’s membrane. | |
| − | + | The formula of sound intensity and its corresponding pressure on cell is below. P is pressure (N/m<sup>2</sup>), I is sound intensity (W/m<sup>2</sup>), ρ is medium density and c is sound velocity. | |
| − | + | {{SUSTech_Shenzhen/bmath|equ=<nowiki>I = \frac{P^{2}}{2c\rho}</nowiki>}} | |
| − | + | == Microfluidics == | |
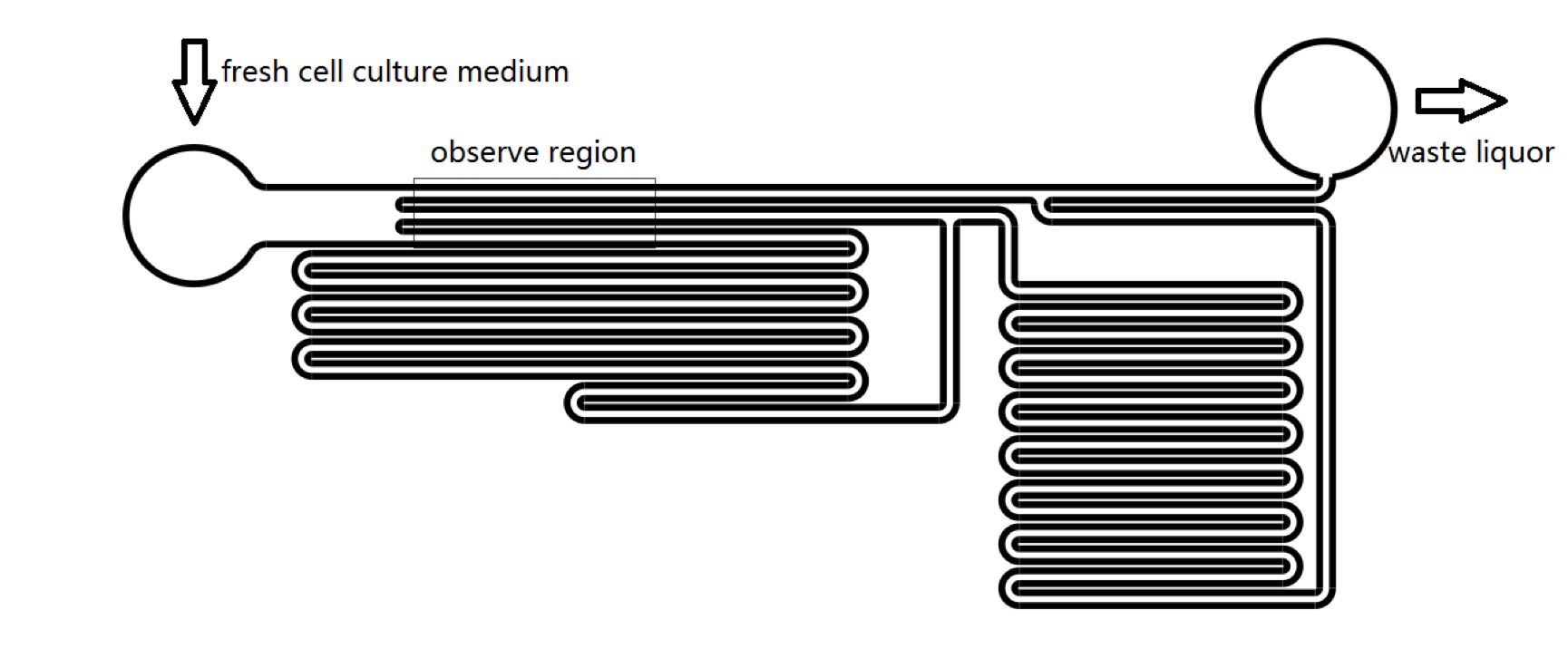
| − | + | We designed microfluidics chips to produce shear stress to stimulate Piezo1 and TRPC5 channel. We could manipulate the pressure on cell membrane by changing the culture medium flowing velocity in microfluidics channels. We have proved that, it could takes less than 1 second for R-GECO to reach maximum fluorescence intensity from receiving stimulus to release fluorescence. With numerical and analytical calculation of fluid dynamics equations, we could predict the sensitivity of MS channels in terms of mechanical stress (Fig. 7), which could be a guidance for sound experiment. We could calculate the amount of pressure that should be applied on cell’s membrane to activate MS channels. (See [[Team:SUSTech_Shenzhen/Model Model]]). | |
| − | == | + | {{SUSTech_Image | filename=T--SUSTech_Shenzhen--F5AE13C9-1AB2-478E-97C6-5E7C8E8A41CD.png | caption=<B>Figure 7. Structure of Microfluidic Chips</B> | width=1000px}} |
| − | + | = 4. Audiogenetics platform construction with directed-evolution = | |
| − | + | With continuing development in synthetic biology, plenty of methods have been developed to regulate gene expression artificially, including the two most common regulation systems, chemical genetics <ref>Nevozhay, D., et al., Negative autoregulation linearizes the dose-response and suppresses the heterogeneity of gene expression. Proc Natl Acad Sci U S A, 2009. 106(13): p. 5123-8.</ref> and optogenetics.<ref>Leifer, A.M., et al., Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods, 2011. 8(2): p. 147-52.</ref> <ref>Ye, H., et al., A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science, 2011. 332(6037): p. 1565-8.</ref> | |
| − | + | In chemical genetics, small molecule drugs such as aTc are used to induce gene expression, while it lacks spatial specificity and the concentration of small chemicals in the cell is not stable. Optogenetics employs light as an input signal to induce gene expression. However, there are side effects caused by laser-induced heating and abnormal ion distribution caused by over-expressed pumps or channels. In addition, undesired disturbance of homeostasis can make it difficult for experimental interpretation. Shallow penetration of light in human tissue also limit the clinical application of optogenetics.<ref>Song, M.Y. and J.X. Yuan, Introduction to TRP channels: structure, function, and regulation. Adv Exp Med Biol, 2010. 661: p. 99-108.</ref> | |
| − | + | Based on the previous study, we hoped to establish a platform to realize audiogenetics. Compared to the methods above, sound signal is easy to generate and less harmful to research objects. | |
| − | + | The key element of this platform is the receptor of sound, which is the mechanosensitive channel. We developed a method to screen channel mutants which could response to specific sound frequency and intensity. | |
| − | + | First of all, a library of TRPC5 channel was constructed by random mutation. We made the ankyrin repeats region (which is proposed to responsible for mechanical force sensing.<ref> Zholos, A.V., Trpc5. Handb Exp Pharmacol, 2014. 222: p. 129-56.</ref> | |
| + | <ref> Beck, A., et al., Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem, 2013. 288(27): p. 19471-83.</ref><ref> Gaudet, R., TRP channels entering the structural era. J Physiol, 2008. 586(15): p. 3565-75.</ref><ref> Owsianik, G., et al., Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol, 2006. 156: p. 61-90</ref><ref> Beck, A., et al., Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem, 2013. 288(27): p. 19471-83.</ref> <ref> Shen, B., et al., Plasma membrane mechanical stress activates TRPC5 channels. PLoS One, 2015. 10(4): p. e0122227.</ref> ) as the mutation region by using error-prone PCR. | ||
| − | |||
| − | + | Secondly, cell with single copy TRPC5 should be produced. As been reported, high-efficiency screening method is crucial for directed evolution. Thus, we need to ensure that each transfected cell has only one mutated TRPC5 copy---cells contain two or more copy number of TRPC5 could complicate the selectivity. To realize this goal, we developed a two-step strategy, used PiggyBac transposon to integrate a single Loxp site into CHO-K1 cells’ genome. Then, we got single colonies by FACS. After extracting cell’s genome, we established a real-time qPCR strategy to identify cell clones with single copy Loxp inserted to genome. With this cell, we then used Cre-loxp system to integrate a single copy of TRPC5 mutant into genome. The diversity of cell library with TRPC5 mutants is also critical. Although the new site-specific recombination method might provide one step solution, however the recombination efficiency of ~1% for CRISPR, is not comparable of the recombination efficiency of 10-80% for Cre-LoxP system. | |
| − | + | Downstream of channels' response is designed to indicate channels’ sensitivity. High sensitivity channels in cells would induce stronger GFP expression through downstream promoter (pNFAT), since there is more calcium influx into cytosol under same condition. | |
| − | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--DZ6.png |width=1000px|caption=<B>Figure 8. A two-step TRPC5 mutant library screening strategy.</B>1. Neo gene with C-terminal Loxp sequence is integrated in to the CHO genome with piggyBac transposon system, and selected with G418. 2. The cell line with a single LoxP integration is used to insert Loxp-puro-TRPC5 library, and selected with puromycin. The cell library is exposed to chronic sonic stimulation, and clones are FACS sorted based on NFAT-GFP intensities.}} | |
= References = | = References = | ||
Latest revision as of 03:46, 20 October 2016

Design
Project
Contents
Overview
For most cells, the molecular mechanism of sensing mechanical force starts with mechanical sensitive (MS) ion channels. The key objective of this study is to explore the possibility of using MS channels to sense sound instead.
Here we adapt synthetic biology approaches to engineer non sensory neuron to detect different aspects of sound, such as frequency and intensity (Fig. 1). Mechanosensitive channels TRPC5 and Piezo1 were chosen as putative receptors of sound. We engineered downstream calcium sensor to visualize channels’ response to sound. Different channels might have different responses even exposed to the same condition. Quantitative characterization of these channels will help us to comprehend the MS channel expression cell as a whole. The downstream NFAT reporter can be used to regulation extrageneous gene expression for further cell function control, as well as for functional selection of mutant channel library.
1. Establish indicator of channels’ response
To visualize channels’ response, we established the systematic characterization tools to analyze the response of MS channel-expression CHO cell to mechanical force. Mechanosensitive channels will induce calcium influx into cells after receiving stimulation. Cytosolic calcium increase is visualized by R-GECO, a genetically encoded calcium sensor which can emit red fluorescence upon calcium binding. Red fluorescence can be captured and analyzed by live cell imaging system.
Mechanosensitive channels: Piezo1 & TRPC5
Piezo1 protein is a functionally diverse mechanosensitive cation channel. It is expressed in lungs, bladder and skin, where mechanosensation plays an important biological role. It also plays significant role in multiple physiological processes, including sensing shear stress of blood flow for proper blood vessel development, regulating red blood cell function and controlling cell migration as well as differentiation. Despite the functional importance and high sensitivity of Piezo1 proteins, their gating mechanisms and three-dimensional (3D) structures are yet to be defined. </ref>Ge, J., et al., Architecture of the mammalian mechanosensitive Piezo1 channel. [1] The density map revealed that Piezo1 formed a three-blade, propeller-shaped architecture, with distinct regions resembling the typical structural components of a propeller, including three blades and a central cap. (full length, 2,547 amino acids) (Fig. 2,3).
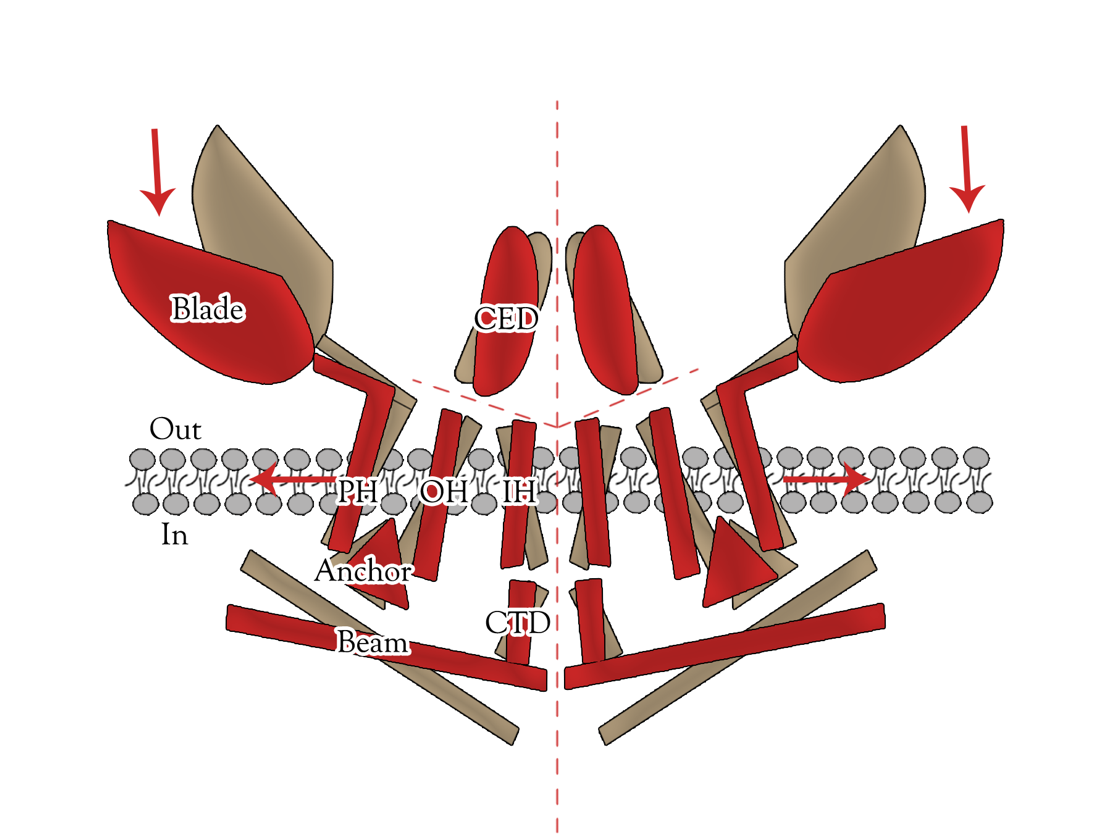
Transient receptor potential (TRP) channels belong to a diverse family of cation channels that respond to a variety of signals. [3][4] TRPC5 is a calcium permeable cation channel predominantly express in the central nervous system (CNS). There is an impressive array of other activators of TRPC5 channel, such as nitric oxide, lysophospholipids, sphingosine-1-phosphate, reduced thioredoxin, protons, lanthanides, and calcium, among them many can cause the change of TRPC5 configuration. Moreover, TRPC5 shows constitutive activity. It is shown to be associated with membrane stretch and cold feeling. Thus, TRPC5 channel has significant potential for synergistic activation and may serve as an important focal point in calcium signaling and electrogenesis. The biological functions of TRPC5 channel are also important, ranging from neurotransmission to control of axon guidance, vascular smooth muscle cell migration and contractility. [5]
TRPC5 shares several common features with other members of the TRP superfamily of ion channels, such as membrane topology and high selectivity to cations over anions. [6][7] The channel core consists of six helical transmembrane (TM1–6) segments linked by extracellular and intracellular segments of variable length and flanked by the cytoplasmic N (Nt, 330 aa)- and C (Ct, 351 aa)-termini(Figure 4). A putative pore region including pore helix between TM5 and TM6 lines the channel pore when a functional channel is formed by assembling four subunits. [8]
There are several general mechanisms for mechanical activation of an ion channel. These include 1) direct channel activation by altering bilayer tension/bending/thickness, 2) indirect channel activation via mechanosensitive signaling molecules, and 3) direct channel activation by tethering to cytoskeletal elements that are exposed to mechanical forces. [9][10] Based on previous study exploring TRP channels gating mechanism, we know that multiple ankyrin repeats in the channel can form a helical structure, which may act as a gating spring. [11] Thus we chose ankyrin repeats as directed mutation region,
Fluorescent component R-GECO (Calcium Indicator)
R-GECO consists of a circularly permuted mApple red FP, which is flanked on one side by the calcium-binding protein calmodulin and on the other side by the calmodulin-binding peptide M13. In the presence of calcium, calmodulin-M13 interactions elicit conformational changes in the fluorophore environment that leads to an increase in the emitted fluorescence. (Fig. 5).[12] They are useful in neuronal activity study and cell imaging for calcium indicator. We could use following methods to induce calcium influx to quantitatively examine the function of R-GECO.
Depolarization
Potassium ion is involved to make a depolarization and lead to the increases in cytosolic free calcium.[13]
Ionomycin
Ionomycin is an ionophore produced by the bacterium Streptomyces conglobatus. It is used in research to raise the intracellular level of calcium (Ca2+) by stimulating store-regulated cation entry. [14]
2. Plasmid construction
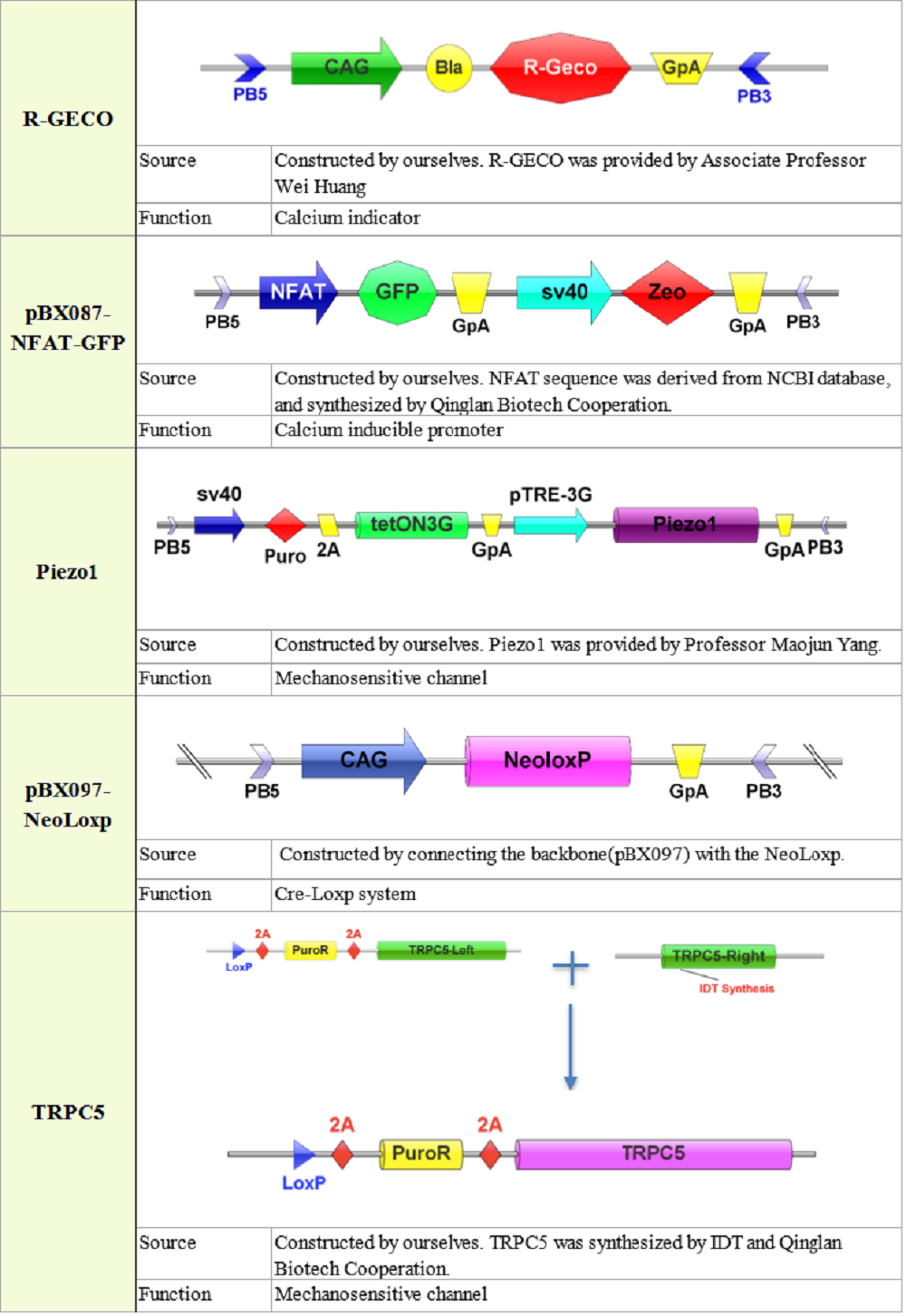
3. Quantitative characterization of Piezo1 and TRPC5
Mechanical stimuli are generated by three ways: 1) Hypoosmolarity; 2) Sonic wave; 3) Microfluidics
To quantitatively characterize these two channels, we employed hypoosmolarity and different magnitude of shear stress to stimulate cells. Besides, we also explored new manipulation using sound with different intensities and frequencies to test cells’ response.
Hypoosmolarity
After serial dilution of medium, we used osmotic pressure to generate mechanical stress exert on cell plasma membrane to activate Piezo1 or TRPC5 channel.
Diluted medium could induce a sharply increase in fluorescence intensity, reveals that cell could sense this stress, in other words, hypoosmolarity can trigger calcium influx through Piezo1 and TRPC5 channel.
Sound
In this part, we supposed that different frequencies and intensities would have great different impact on cells, so we employed a wide range of frequency and intensity of sound to stimulate the cell. We used home-made sound generators utilizing piezo buzzers, balanced armatures, speakers, ultrasound transducers and atomisers to activate Piezo1 and TRPC5 channel. Fluorescence intensity increase could be observed after intracellular calcium concentration increased. There is little literature report of quantitive research on channels employing sound as stimulus, we wanted to quantify our experiment, simulation and calculation of how much energy we should apply on cell’s membrane.
The formula of sound intensity and its corresponding pressure on cell is below. P is pressure (N/m2), I is sound intensity (W/m2), ρ is medium density and c is sound velocity.
I = \frac{P^{2}}{2c\rho}
Microfluidics
We designed microfluidics chips to produce shear stress to stimulate Piezo1 and TRPC5 channel. We could manipulate the pressure on cell membrane by changing the culture medium flowing velocity in microfluidics channels. We have proved that, it could takes less than 1 second for R-GECO to reach maximum fluorescence intensity from receiving stimulus to release fluorescence. With numerical and analytical calculation of fluid dynamics equations, we could predict the sensitivity of MS channels in terms of mechanical stress (Fig. 7), which could be a guidance for sound experiment. We could calculate the amount of pressure that should be applied on cell’s membrane to activate MS channels. (See Team:SUSTech_Shenzhen/Model Model).
4. Audiogenetics platform construction with directed-evolution
With continuing development in synthetic biology, plenty of methods have been developed to regulate gene expression artificially, including the two most common regulation systems, chemical genetics [15] and optogenetics.[16] [17]
In chemical genetics, small molecule drugs such as aTc are used to induce gene expression, while it lacks spatial specificity and the concentration of small chemicals in the cell is not stable. Optogenetics employs light as an input signal to induce gene expression. However, there are side effects caused by laser-induced heating and abnormal ion distribution caused by over-expressed pumps or channels. In addition, undesired disturbance of homeostasis can make it difficult for experimental interpretation. Shallow penetration of light in human tissue also limit the clinical application of optogenetics.[18]
Based on the previous study, we hoped to establish a platform to realize audiogenetics. Compared to the methods above, sound signal is easy to generate and less harmful to research objects.
The key element of this platform is the receptor of sound, which is the mechanosensitive channel. We developed a method to screen channel mutants which could response to specific sound frequency and intensity.
First of all, a library of TRPC5 channel was constructed by random mutation. We made the ankyrin repeats region (which is proposed to responsible for mechanical force sensing.[19] [20][21][22][23] [24] ) as the mutation region by using error-prone PCR.
Secondly, cell with single copy TRPC5 should be produced. As been reported, high-efficiency screening method is crucial for directed evolution. Thus, we need to ensure that each transfected cell has only one mutated TRPC5 copy---cells contain two or more copy number of TRPC5 could complicate the selectivity. To realize this goal, we developed a two-step strategy, used PiggyBac transposon to integrate a single Loxp site into CHO-K1 cells’ genome. Then, we got single colonies by FACS. After extracting cell’s genome, we established a real-time qPCR strategy to identify cell clones with single copy Loxp inserted to genome. With this cell, we then used Cre-loxp system to integrate a single copy of TRPC5 mutant into genome. The diversity of cell library with TRPC5 mutants is also critical. Although the new site-specific recombination method might provide one step solution, however the recombination efficiency of ~1% for CRISPR, is not comparable of the recombination efficiency of 10-80% for Cre-LoxP system.
Downstream of channels' response is designed to indicate channels’ sensitivity. High sensitivity channels in cells would induce stronger GFP expression through downstream promoter (pNFAT), since there is more calcium influx into cytosol under same condition.
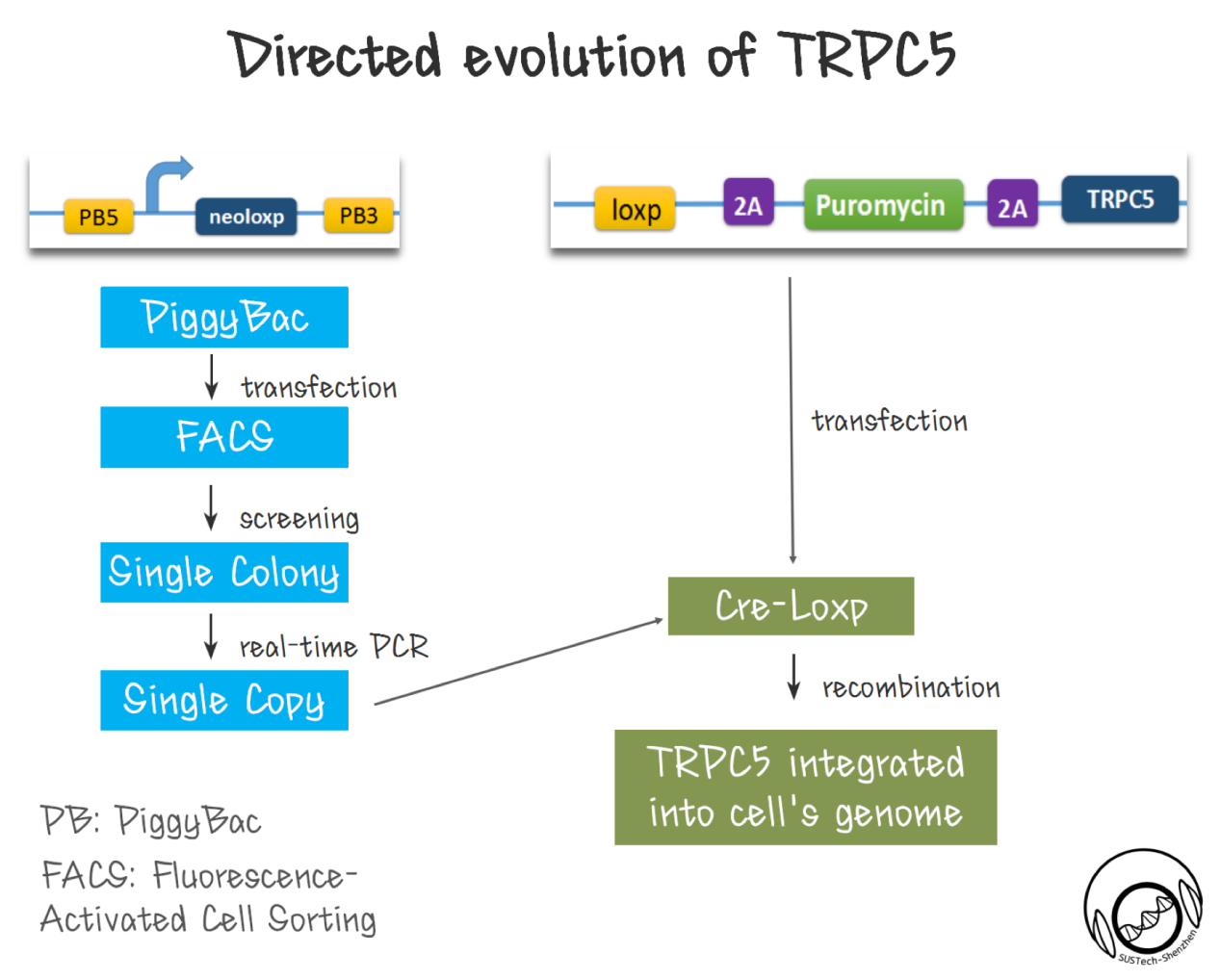
References
- ↑ Nature, 2015. 527(7576): p. 64-9.
- ↑ Ge, J., et al., Architecture of the mammalian mechanosensitive Piezo1 channel. Nature, 2015. 527(7576): p. 64-9.
- ↑ Flockerzi, V., An introduction on TRP channels. Handb Exp Pharmacol, 2007(179): p. 1-19.
- ↑ Ramsey, I.S., M. Delling, and D.E. Clapham, An introduction to TRP channels. Annu Rev Physiol, 2006. 68: p. 619-47.
- ↑ Zholos, A.V., Trpc5. Handb Exp Pharmacol, 2014. 222: p. 129-56.
- ↑ Owsianik G, Talavera K, Voets T et al (2006) Permeation and selectivity of TRP channels. Annu Rev Physiol 68:685–717.
- ↑ Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647
- ↑ B. Nilius and V. Flockerzi (eds.), Mammalian Transient Receptor Potential (TRP) Cation Channels, Handbook of Experimental Pharmacology 222.
- ↑ Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007; 428:183–207. Epub 2007/09/19.
- ↑ Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007; 8(7):510–21. Epub 2007/06/23.
- ↑ B. Nilius and V. Flockerzi (eds.), Mammalian Transient Receptor Potential (TRP) Cation Channels, Handbook of Experimental Pharmacology 222.
- ↑ Zhao, Y., et al., An expanded palette of genetically encoded Ca(2)(+) indicators. Science, 2011. 333(6051): p. 1888-91.
- ↑ Ranade, S.S., et al., Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A, 2014. 111(28): p. 10347-52.
- ↑ Morgan, A.J. and R. Jacob, Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J, 1994. 300 ( Pt 3): p. 665-72..
- ↑ Nevozhay, D., et al., Negative autoregulation linearizes the dose-response and suppresses the heterogeneity of gene expression. Proc Natl Acad Sci U S A, 2009. 106(13): p. 5123-8.
- ↑ Leifer, A.M., et al., Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods, 2011. 8(2): p. 147-52.
- ↑ Ye, H., et al., A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science, 2011. 332(6037): p. 1565-8.
- ↑ Song, M.Y. and J.X. Yuan, Introduction to TRP channels: structure, function, and regulation. Adv Exp Med Biol, 2010. 661: p. 99-108.
- ↑ Zholos, A.V., Trpc5. Handb Exp Pharmacol, 2014. 222: p. 129-56.
- ↑ Beck, A., et al., Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem, 2013. 288(27): p. 19471-83.
- ↑ Gaudet, R., TRP channels entering the structural era. J Physiol, 2008. 586(15): p. 3565-75.
- ↑ Owsianik, G., et al., Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol, 2006. 156: p. 61-90
- ↑ Beck, A., et al., Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem, 2013. 288(27): p. 19471-83.
- ↑ Shen, B., et al., Plasma membrane mechanical stress activates TRPC5 channels. PLoS One, 2015. 10(4): p. e0122227.