JackieLiao (Talk | contribs) |
JackieLiao (Talk | contribs) |
||
| (44 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
<html> | <html> | ||
<meta http-equiv="X-UA-Compatible" content="IE=edge"> | <meta http-equiv="X-UA-Compatible" content="IE=edge"> | ||
| − | |||
| − | |||
<style> | <style> | ||
#sideMenu | #sideMenu | ||
| Line 16: | Line 14: | ||
#content { padding:0px; width:100%; margin-left:0%; margin-right:0%;} | #content { padding:0px; width:100%; margin-left:0%; margin-right:0%;} | ||
</style> | </style> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- ////////////////////// START TOP HEADER /////////////////////// --> | <!-- ////////////////////// START TOP HEADER /////////////////////// --> | ||
<head> | <head> | ||
| − | |||
| − | |||
<style> | <style> | ||
body { | body { | ||
| Line 32: | Line 22: | ||
.top { | .top { | ||
position: absolute; | position: absolute; | ||
| − | top: - | + | top: -1%; |
| − | left: | + | left: 0%; |
| − | + | ||
width: 100%; | width: 100%; | ||
| − | height: | + | box-sizing:border-box; |
| + | height: 115px; | ||
font-family: Corbel; | font-family: Corbel; | ||
| − | overflow: visible; | + | overflow:visible; |
| + | z-index: +1; | ||
} | } | ||
.mid { | .mid { | ||
position: absolute; | position: absolute; | ||
| − | left: | + | left: 5%; |
| − | width: | + | width: 80%; |
| − | height: | + | height: 100%; |
font-family: Corbel; | font-family: Corbel; | ||
| − | |||
} | } | ||
.headline { | .headline { | ||
| Line 55: | Line 45: | ||
} | } | ||
.header-subnav { | .header-subnav { | ||
| − | background: # | + | background: #0D014D; |
width: 100%; | width: 100%; | ||
position: absolute; | position: absolute; | ||
text-align: center; | text-align: center; | ||
margin: 0 auto; | margin: 0 auto; | ||
| − | top: | + | top: 116%; |
| − | left: | + | left: 0%; |
font-family: Corbel; | font-family: Corbel; | ||
| + | z-index:-1; | ||
} | } | ||
.header-subnav li { | .header-subnav li { | ||
| Line 94: | Line 85: | ||
.circle { | .circle { | ||
position:absolute; | position:absolute; | ||
| − | left: | + | left: 85.5%; |
| − | top: | + | top: 70%; |
width: 180px; | width: 180px; | ||
height: 180px; | height: 180px; | ||
| − | background: # | + | background: #0D014D; |
-moz-border-radius: 90px; | -moz-border-radius: 90px; | ||
-webkit-border-radius: 90px; | -webkit-border-radius: 90px; | ||
| Line 105: | Line 96: | ||
.left { | .left { | ||
position: fixed; | position: fixed; | ||
| − | left: | + | left: 25px; |
| − | top: | + | top: 230px; |
font-family: Corbel; | font-family: Corbel; | ||
background: rgba(250, 250, 250, 0.15); | background: rgba(250, 250, 250, 0.15); | ||
| − | width: | + | width: 170px; |
z-index:+1; | z-index:+1; | ||
} | } | ||
| Line 162: | Line 153: | ||
width: 600px; | width: 600px; | ||
height: 500px; | height: 500px; | ||
| − | |||
| − | |||
| − | |||
| − | |||
position: absolute; | position: absolute; | ||
z-index: 1; | z-index: 1; | ||
| Line 255: | Line 242: | ||
background-color: #FF8800; | background-color: #FF8800; | ||
height: 2px; | height: 2px; | ||
| + | } | ||
| + | .list li { | ||
| + | list-style:none; | ||
| + | background-image:url(https://static.igem.org/mediawiki/2016/b/ba/CGU_Taiwan--point.jpg); | ||
| + | background-repeat:no-repeat; | ||
| + | background-position:-10 5px; | ||
| + | padding-left:3%; | ||
| + | background-size:2.5%; | ||
| + | } | ||
| + | |||
| + | .end2 { | ||
| + | background-color:#0D014D; | ||
| + | position: absolute; | ||
| + | width: 100%; | ||
| + | height: 285%; | ||
| + | font-family: Corbel; | ||
| + | z-index:+1; | ||
| + | } | ||
| + | .sitemap { | ||
| + | background-color:#0D014D; | ||
| + | position: absolute; | ||
| + | width: 20%; | ||
| + | height: 40%; | ||
| + | font-family: Corbel; | ||
| + | } | ||
| + | .list1 li { | ||
| + | list-style:none; | ||
| + | background-image:url(https://static.igem.org/mediawiki/2016/5/58/CGU_Taiwan--sitemap.jpg); | ||
| + | background-repeat:no-repeat; | ||
| + | background-position:10px; | ||
| + | background-size:9%; | ||
| + | margin-bottom:-20%; | ||
| + | } | ||
| + | .list2 li { | ||
| + | list-style:none; | ||
| + | background-image:url(https://static.igem.org/mediawiki/2016/2/2f/CGU_Taiwan--sitemap2.jpg); | ||
| + | background-repeat:no-repeat; | ||
| + | background-position:6px; | ||
| + | background-size:7%; | ||
| + | margin-bottom:-10%; | ||
| + | } | ||
| + | .list3 li { | ||
| + | list-style:none; | ||
| + | background-image:url(https://static.igem.org/mediawiki/2016/3/31/CGU_Taiwan--sitemap3.jpg); | ||
| + | background-repeat:no-repeat; | ||
| + | background-position:5%; | ||
| + | background-size:2%; | ||
| + | margin-bottom:-10%; | ||
} | } | ||
</style> | </style> | ||
| Line 307: | Line 342: | ||
<body> | <body> | ||
| − | |||
| − | |||
| − | |||
<a name='anchor0'></a> | <a name='anchor0'></a> | ||
| − | <div class=" | + | <div class="top"> |
| + | <img style="margin-top:-2%;width:100%;height:140%;position:absolute;" src="https://static.igem.org/mediawiki/2016/0/0b/CGU_Taiwan--top.jpg"> | ||
| + | </div> | ||
</div> | </div> | ||
<header class="top"> | <header class="top"> | ||
| − | |||
<ul class="header-subnav"> | <ul class="header-subnav"> | ||
<li><a href="https://2016.igem.org/Team:CGU_Taiwan">HOME</a></li> | <li><a href="https://2016.igem.org/Team:CGU_Taiwan">HOME</a></li> | ||
| Line 327: | Line 360: | ||
<li><a href="https://2016.igem.org/Team:CGU_Taiwan/Parts">PARTS</a></li> | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Parts">PARTS</a></li> | ||
</ul> | </ul> | ||
| + | <div class="circle"><img style="position:absolute;z-index:+1;width:90%;height:95%;margin-left:3%;margin-top:2%;" src="https://static.igem.org/mediawiki/2016/8/8a/CGU_Taiwan--logo9.jpg"></div> | ||
</header> | </header> | ||
| − | |||
| − | < | + | <div class="mid" style="margin-left:5%;margin-top:20%;"> |
| + | <br> | ||
| + | <div style="font-size:60px;color: #CE0000;text-decoration:none;"> | ||
Safety | Safety | ||
| − | </ | + | </div> |
| + | <br><br><br><br> | ||
| − | (1)Short introduction of our project | + | <b style="color:#0D014D;font-size:20px;">(1) Short introduction of our project</b> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
Transgenic Leishmania that can be inactivated by light exposure acts as a safe carrier to deliver specific antigens to the APCs for T cells and humoral response. Based on this concept, we established a new model system to generate antigen-specific inactive Leishmania adjuvant (Leijuvant). | Transgenic Leishmania that can be inactivated by light exposure acts as a safe carrier to deliver specific antigens to the APCs for T cells and humoral response. Based on this concept, we established a new model system to generate antigen-specific inactive Leishmania adjuvant (Leijuvant). | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | (2)What organisms do we use? | + | <b style="color:#0D014D;font-size:20px;">(2) What organisms do we use? </b> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
Leishmania, DC2.4, E.coli(DH5α, BL21), C57BL/6 mice | Leishmania, DC2.4, E.coli(DH5α, BL21), C57BL/6 mice | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(3) Which part do we use? </b> | |
| − | (3)Which part do we use? | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
Full length of HA and OVA: We used full length sequence of hemagglutinin (HA) of H1N1 and Ovalbumin as our testing antigen that is expressed in Leishmania. <br> | Full length of HA and OVA: We used full length sequence of hemagglutinin (HA) of H1N1 and Ovalbumin as our testing antigen that is expressed in Leishmania. <br> | ||
5’UTR, 3’UTR, 2.3kb intron: the sequence is originally from Leishmania genome. Act as promotor, terminator, and expression modulating part in our device. | 5’UTR, 3’UTR, 2.3kb intron: the sequence is originally from Leishmania genome. Act as promotor, terminator, and expression modulating part in our device. | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(4) Where do we perform the experiments?</b> | |
| − | (4)Where do we perform the experiments? | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
Leishmania and DC2.4 culturing and experiments will be performed in a BSL-2 lab within the flow hood in an independent room and requires wearing lab gloves. Once the Leishmania is inactivated it can be manipulated on the open bench. DH5α and BL21 are manipulated in the Laminar flow. Normal experiments are performed on open bench with gloves. | Leishmania and DC2.4 culturing and experiments will be performed in a BSL-2 lab within the flow hood in an independent room and requires wearing lab gloves. Once the Leishmania is inactivated it can be manipulated on the open bench. DH5α and BL21 are manipulated in the Laminar flow. Normal experiments are performed on open bench with gloves. | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(5) The training of the lab team</b> | |
| − | (5)The training of the lab team | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
We received lecture of the following topics: registration for the use of biological materials, human pathogens, recombinant DNA experiments, potentially infectious materials, animals handling, the use of biological safety cabinets (BSCs) and other laminar flow benches (LFBs), emergency procedures for incidents, biosafety levels signs and labels, etc. We further received laboratory practices training of biosafety level 1 and 2. All the experiments are under Dr. Yu and Dr. Chang’s instructions. | We received lecture of the following topics: registration for the use of biological materials, human pathogens, recombinant DNA experiments, potentially infectious materials, animals handling, the use of biological safety cabinets (BSCs) and other laminar flow benches (LFBs), emergency procedures for incidents, biosafety levels signs and labels, etc. We further received laboratory practices training of biosafety level 1 and 2. All the experiments are under Dr. Yu and Dr. Chang’s instructions. | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(6) How do we protect us? </b> | |
| − | (6)How do we protect us? | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
Follow the requirements from different biosafety level laboratory strictly, including wearing gloves, lab coats, masks and goggles if required. | Follow the requirements from different biosafety level laboratory strictly, including wearing gloves, lab coats, masks and goggles if required. | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(7) Safety concern in our project</b> | |
| − | (7)Safety concern in our project | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
Leishmania is a genus of trypanosomes that are responsible for the disease leishmaniasis, a blood-borne disease that is spread by sandflies. Leishmania culturing and experiments will be performed in a BSL-2 lab within the flow hood in an independent room and requires wearing lab gloves. There is almost no chance of infecting people under this experimental condition since there is no direct contact with Leishmania. Also, Leishmania will not be able to survive once it leaves the culture condition. Once it is photo-inactivated it can be manipulated on the open bench. | Leishmania is a genus of trypanosomes that are responsible for the disease leishmaniasis, a blood-borne disease that is spread by sandflies. Leishmania culturing and experiments will be performed in a BSL-2 lab within the flow hood in an independent room and requires wearing lab gloves. There is almost no chance of infecting people under this experimental condition since there is no direct contact with Leishmania. Also, Leishmania will not be able to survive once it leaves the culture condition. Once it is photo-inactivated it can be manipulated on the open bench. | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(8) How to verify the safety of leishmania after photo-inactivation</b> | |
| − | (8)How to verify the safety of leishmania after photo-inactivation | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
1. How to conduct the photo-inactivation? | 1. How to conduct the photo-inactivation? | ||
</div> | </div> | ||
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:120px;"> |
Photosensitizers (PS) can be delivered endogenously with delta-aminolevulinate (ALA) and exogenously with phthalocyanines (PC). | Photosensitizers (PS) can be delivered endogenously with delta-aminolevulinate (ALA) and exogenously with phthalocyanines (PC). | ||
Detailed protocol (link)<br> | Detailed protocol (link)<br> | ||
| − | With loading ALA, we can see the florescence with the excitation of uroporphyrin in the 12-well tissue culture plate during illumination of long-wave UV lamp (365nm)1(Fig. 1A.). There wasn’t any illumination by UV lamp without loading ALA(Fig. 1B.). After illuminating with long-wave UV , After long-wave UV illumination, we then put it under the LED red light with the wavelength of 620~630nm for 10 minutes (Fig. 1C.) and the double photo inactivation is complete. | + | With loading ALA, we can see the florescence with the excitation of uroporphyrin in the 12-well tissue culture plate during illumination of long-wave UV lamp (365nm)1(Fig. 1A.). There wasn’t any illumination by UV lamp without loading ALA(Fig. 1B.). After illuminating with long-wave UV , After long-wave UV illumination, we then put it under the LED red light with the wavelength of 620~630nm for 10 minutes (Fig. 1C.) and the double photo inactivation is complete. <br><br> |
| + | <img src="https://static.igem.org/mediawiki/2016/f/f1/LeishSafety1.png" width=550px height=400px style="border:2px black solid;border-radius:8px;"></img> | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> | |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
2. How to confirm the sucess of photo-inactivation?<br> | 2. How to confirm the sucess of photo-inactivation?<br> | ||
| − | According to (Chang, K. P., et al. 2016.)2we can use the following method to confirm the safety of photo-inactivated Leishmania. | + | <font size=2>According to (Chang, K. P., et al. 2016.)2we can use the following method to confirm the safety of photo-inactivated Leishmania.</font> |
</div> | </div> | ||
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:120px;"> |
| − | + | <b> (I) Microscopy of flagellar motility</b> | |
</div> | </div> | ||
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:170px;"> |
After illuminating by long-wave UV and red light, you can observe the samples under the microscope. The difference between [+drug, +light]; [+drug, -light]; [-drug;-light] groups can be easily seen. With the illumination of the both chemicals, all six wells of one plate show no flagellar motility under 100x magnification. However, the control group, [+drug, -light] and [-drug;-light], have high motility same as the wild-type Leishmania. | After illuminating by long-wave UV and red light, you can observe the samples under the microscope. The difference between [+drug, +light]; [+drug, -light]; [-drug;-light] groups can be easily seen. With the illumination of the both chemicals, all six wells of one plate show no flagellar motility under 100x magnification. However, the control group, [+drug, -light] and [-drug;-light], have high motility same as the wild-type Leishmania. | ||
</div> | </div> | ||
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:120px;"> |
| − | (II) Cultivation for growth (2 weeks) | + | <b>(II) Cultivation for growth (2 weeks)</b> |
</div> | </div> | ||
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:170px;"> |
To further confirm the complete inactivation of Leishmania, we separated 100μl from each sample, [+drug, +light], [+drug, -light] and [-drug;-light], right after illumination. And add them into 24-Well plate containing M199+10%HI-FB medium. Observed and kept tract of it continuously for two weeks. Except the [+drug, +light] group, the other two groups were moving and increasing normally. As for the [+drug, +light] group, there was no motility, or proliferation of the Leishmania from the beginning to the end of the two weeks. | To further confirm the complete inactivation of Leishmania, we separated 100μl from each sample, [+drug, +light], [+drug, -light] and [-drug;-light], right after illumination. And add them into 24-Well plate containing M199+10%HI-FB medium. Observed and kept tract of it continuously for two weeks. Except the [+drug, +light] group, the other two groups were moving and increasing normally. As for the [+drug, +light] group, there was no motility, or proliferation of the Leishmania from the beginning to the end of the two weeks. | ||
| + | <br><br><br> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/c/cd/CGU_Taiwan--GIF-1.gif" width=200px height=200px></img> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/8/83/CGU_Taiwan--GIF-2.gif" width=200px height=200px></img> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/0/06/CGU_Taiwan--GIF-3.gif" width=200px height=200px></img> | ||
| + | <br>(From left to right: [+drug,+light] [+drug,-light] [-drug,-light]) | ||
| + | <br><br> | ||
</div> | </div> | ||
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:120px;"> |
| − | + | <b> (III) 2 months after subcutaneously injecting inactivated Leishmania into mice</b> | |
</div> | </div> | ||
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:170px;"> |
| − | We subcutaneously injected mouse with108 of photo-inactivated Leishmania on both sides of the back around the shoulders, and injected normal saline as the control group. There was no skin ulcers or inflammation and no any other Leishmaniasis symptoms among the 10 mouse that serve as our experimental group | + | We subcutaneously injected mouse with108 of photo-inactivated Leishmania on both sides of the back around the shoulders, and injected normal saline as the control group. There was no skin ulcers or inflammation and no any other Leishmaniasis symptoms among the 10 mouse that serve as our experimental group. <br><br> |
</div> | </div> | ||
| + | <br> | ||
| + | <div style="color:black;text-decoration:none;font-size:12px;margin-left:170px;"> | ||
| + | Reference:<br> | ||
| + | 1. Dutta, S., et al. (2008). "Transgenic Leishmania model for delta-aminolevulinate-inducible monospecific uroporphyria: cytolytic phototoxicity initiated by singlet oxygen-mediated inactivation of proteins and its ablation by endosomal mobilization of cytosolic uroporphyrin." Eukaryot Cell 7(7): 1146-1157.<br> | ||
| + | 2. Chang, K. P., et al. (2016). "New "light" for one-world approach toward safe and effective control of animal diseases and insect vectors from leishmaniac perspectives." Parasit Vectors 9(1): 396. | ||
| + | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(9) The product safety concern in the future</b> | |
| − | (9) The product safety concern in the future | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | <u>Environmental concern</u><br> |
| − | Environmental concern<br> | + | |
Leishmania culture requires a certain osmolarity and combination of nutrients. Therefore, our transgenic Leishmania will not survive as soon as it leaves the medium.<br> | Leishmania culture requires a certain osmolarity and combination of nutrients. Therefore, our transgenic Leishmania will not survive as soon as it leaves the medium.<br> | ||
| − | Side effects of adjuvant <br> | + | <u>Side effects of adjuvant </u><br> |
There are some potential adverse effects underlying every drug and vaccine. More animal test should be carried out and the most efficient dose in human will be selected before clinical trials. In the clinical study, side effect after immunization will be identified and study design will be modified as well. If our product passes the FDA approval, the report of unexpected effect will be always under inspection.<br> | There are some potential adverse effects underlying every drug and vaccine. More animal test should be carried out and the most efficient dose in human will be selected before clinical trials. In the clinical study, side effect after immunization will be identified and study design will be modified as well. If our product passes the FDA approval, the report of unexpected effect will be always under inspection.<br> | ||
</div> | </div> | ||
| + | <br><br> | ||
| − | + | <b style="color:#0D014D;font-size:20px;">(10) How would your project be used in the real world?</b> | |
| − | (10) How would your project be used in the real world? | + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> |
| − | <div style="color:black;text-decoration:none;font-size:18px;margin-left: | + | |
Our Leijuvant can act to facilitate both humeral and cellular immune response which may contribute to the development of vaccine nowadays. Combined with our prediction software, the platform can predict the peptides on MHC molecules and help to optimize the peptide presentation which may benefit to engineer the sequence for further research and developing a more efficient vaccine. | Our Leijuvant can act to facilitate both humeral and cellular immune response which may contribute to the development of vaccine nowadays. Combined with our prediction software, the platform can predict the peptides on MHC molecules and help to optimize the peptide presentation which may benefit to engineer the sequence for further research and developing a more efficient vaccine. | ||
</div> | </div> | ||
| + | <br><br> | ||
| + | <b style="color:#0D014D;font-size:20px;">(11)Animal Welfare</b> | ||
| + | <div style="color:black;text-decoration:none;font-size:18px;margin-left:70px;"> | ||
| + | All animals were housed in groups of 4–5 per cage in a room maintained at 22 ± 2 °C with 12 h light/ dark cycle (light on at 7:00 a.m. and off at 7:00 p.m.). Food and water were available <i>ad libitum</i>. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Chang Gung University. | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <div class="end2" style="margin-top:255%;"> | ||
| + | <br> | ||
| + | <ul class="list3" class="margin-left:1%;margin-top:2%;"> | ||
| + | <li><a href="#anchor0"><b style="color:white;font-size:28px;margin-left:7%;">TOP</a></li> | ||
| + | </ul> | ||
| + | <br> | ||
| + | <div class="sitemap" style="margin-left:5%;"> | ||
| + | <br> | ||
| + | <b style="color:white;font-size:28px;margin-top:5%;margin-left:4%;text-decoration:none;">SITE MAP</b><br> | ||
| + | <br><br> | ||
| + | <img style="background-color:#0D014D;margin-left:20%;border-radius:50%;border:6px solid white;padding:5px;width:40%;height:70%;position:absolute;margin-left:2%;" src="https://static.igem.org/mediawiki/2016/0/03/CGU_Taiwan--leishmania3.jpg"> | ||
| + | </div> | ||
| + | <br> | ||
| + | |||
| + | <div class="sitemap" style="margin-left:22%;margin-top:-1%;"> | ||
| + | <ul class="list1" class="margin-left:5%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan"><b style="color:white;font-size:18px;margin-left:20%;">HOME</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Achievements"><b style="color:white;font-size:18px;margin-left:20%;">ACHIEVEMENTS</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Interlab"><b style="color:white;font-size:18px;margin-left:20%;">INTERLAB</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Safety"><b style="color:white;font-size:18px;margin-left:20%;">SAFETY</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Parts"><b style="color:white;font-size:18px;margin-left:20%;">PARTS</li></a> | ||
| + | </ul> | ||
| + | </div> | ||
| + | |||
| + | <div class="sitemap" style="margin-left:40%;margin-top:-2%;"> | ||
| + | <ul class="list1" class="margin-left:5%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Description"><b style="color:white;font-size:18px;margin-left:20%;">PROJECT</li></a> | ||
| + | <br><br> | ||
| + | <ul class="list2" class="margin-left:6%;margin-top:50%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Design"><b style="color:white;font-size:17px;margin-left:10%;">Design</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Background"><b style="color:white;font-size:17px;margin-left:10%;">Background</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Results"><b style="color:white;font-size:17px;margin-left:10%;">Results</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Proof"><b style="color:white;font-size:17px;margin-left:10%;">Proof</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Notebook"><b style="color:white;font-size:17px;margin-left:10%;">Notebook</li></a> | ||
| + | <br><br> | ||
| + | </ul> | ||
| + | </ul> | ||
| + | </div> | ||
| + | |||
| + | <div class="sitemap" style="margin-left:55%;margin-top:-3.5%;"> | ||
| + | <ul class="list1" class="margin-left:5%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Human_Practices"><b style="color:white;font-size:18px;margin-left:20%;">HUMAN PRACTICES</li></a> | ||
| + | <br><br> | ||
| + | <ul class="list2" class="margin-left:6%;margin-top:50%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Human_Practices_Survey"><b style="color:white;font-size:17px;margin-left:10%;">Survey</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Human_Practices_Education"><b style="color:white;font-size:17px;margin-left:10%;">Education</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Human_Practices_Communication"><b style="color:white;font-size:17px;margin-left:10%;">Communication</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Entrepreneurship"><b style="color:white;font-size:17px;margin-left:10%;">Entrepreneurship</li></a> | ||
| + | </ul> | ||
| + | </ul> | ||
| + | </div> | ||
| + | |||
| + | <div class="sitemap" style="margin-left:75%;margin-top:-3.5%;"> | ||
| + | <ul class="list1" class="margin-left:5%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Software"><b style="color:white;font-size:18px;margin-left:20%;">MODELING</li></a> | ||
| + | <br><br> | ||
| + | <ul class="list2" class="margin-left:6%;"> | ||
| + | <li><a href="http://163.25.92.36/igemcgu/igemwebhome.htm"><b style="color:white;font-size:17px;margin-left:10%;">McHug</li></a> | ||
| + | </ul> | ||
| + | </ul> | ||
| + | <ul class="list1" class="margin-left:1%;margin-top:-10%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Team"><b style="color:white;font-size:18px;margin-left:20%;">OUR TEAM</li></a> | ||
| + | <br><br> | ||
| + | <ul class="list2" class="margin-left:6%;margin-top:-5%;"> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Collaborations"><b style="color:white;font-size:17px;margin-left:10%;">Collaborations</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Attributions"><b style="color:white;font-size:17px;margin-left:10%;">Attributions</li></a> | ||
| + | <li><a href="https://2016.igem.org/Team:CGU_Taiwan/Team#anchor2"><b style="color:white;font-size:17px;margin-left:10%;">Sponsors</li></a> | ||
| + | </ul> | ||
| + | </ul> | ||
| + | </div> | ||
</div> | </div> | ||
</body> | </body> | ||
</html> | </html> | ||
Latest revision as of 08:28, 2 December 2016


Safety
(1) Short introduction of our project
Transgenic Leishmania that can be inactivated by light exposure acts as a safe carrier to deliver specific antigens to the APCs for T cells and humoral response. Based on this concept, we established a new model system to generate antigen-specific inactive Leishmania adjuvant (Leijuvant).
(2) What organisms do we use?
Leishmania, DC2.4, E.coli(DH5α, BL21), C57BL/6 mice
(3) Which part do we use?
Full length of HA and OVA: We used full length sequence of hemagglutinin (HA) of H1N1 and Ovalbumin as our testing antigen that is expressed in Leishmania.
5’UTR, 3’UTR, 2.3kb intron: the sequence is originally from Leishmania genome. Act as promotor, terminator, and expression modulating part in our device.
5’UTR, 3’UTR, 2.3kb intron: the sequence is originally from Leishmania genome. Act as promotor, terminator, and expression modulating part in our device.
(4) Where do we perform the experiments?
Leishmania and DC2.4 culturing and experiments will be performed in a BSL-2 lab within the flow hood in an independent room and requires wearing lab gloves. Once the Leishmania is inactivated it can be manipulated on the open bench. DH5α and BL21 are manipulated in the Laminar flow. Normal experiments are performed on open bench with gloves.
(5) The training of the lab team
We received lecture of the following topics: registration for the use of biological materials, human pathogens, recombinant DNA experiments, potentially infectious materials, animals handling, the use of biological safety cabinets (BSCs) and other laminar flow benches (LFBs), emergency procedures for incidents, biosafety levels signs and labels, etc. We further received laboratory practices training of biosafety level 1 and 2. All the experiments are under Dr. Yu and Dr. Chang’s instructions.
(6) How do we protect us?
Follow the requirements from different biosafety level laboratory strictly, including wearing gloves, lab coats, masks and goggles if required.
(7) Safety concern in our project
Leishmania is a genus of trypanosomes that are responsible for the disease leishmaniasis, a blood-borne disease that is spread by sandflies. Leishmania culturing and experiments will be performed in a BSL-2 lab within the flow hood in an independent room and requires wearing lab gloves. There is almost no chance of infecting people under this experimental condition since there is no direct contact with Leishmania. Also, Leishmania will not be able to survive once it leaves the culture condition. Once it is photo-inactivated it can be manipulated on the open bench.
(8) How to verify the safety of leishmania after photo-inactivation
1. How to conduct the photo-inactivation?
Photosensitizers (PS) can be delivered endogenously with delta-aminolevulinate (ALA) and exogenously with phthalocyanines (PC).
Detailed protocol (link)
With loading ALA, we can see the florescence with the excitation of uroporphyrin in the 12-well tissue culture plate during illumination of long-wave UV lamp (365nm)1(Fig. 1A.). There wasn’t any illumination by UV lamp without loading ALA(Fig. 1B.). After illuminating with long-wave UV , After long-wave UV illumination, we then put it under the LED red light with the wavelength of 620~630nm for 10 minutes (Fig. 1C.) and the double photo inactivation is complete.

With loading ALA, we can see the florescence with the excitation of uroporphyrin in the 12-well tissue culture plate during illumination of long-wave UV lamp (365nm)1(Fig. 1A.). There wasn’t any illumination by UV lamp without loading ALA(Fig. 1B.). After illuminating with long-wave UV , After long-wave UV illumination, we then put it under the LED red light with the wavelength of 620~630nm for 10 minutes (Fig. 1C.) and the double photo inactivation is complete.

2. How to confirm the sucess of photo-inactivation?
According to (Chang, K. P., et al. 2016.)2we can use the following method to confirm the safety of photo-inactivated Leishmania.
According to (Chang, K. P., et al. 2016.)2we can use the following method to confirm the safety of photo-inactivated Leishmania.
(I) Microscopy of flagellar motility
After illuminating by long-wave UV and red light, you can observe the samples under the microscope. The difference between [+drug, +light]; [+drug, -light]; [-drug;-light] groups can be easily seen. With the illumination of the both chemicals, all six wells of one plate show no flagellar motility under 100x magnification. However, the control group, [+drug, -light] and [-drug;-light], have high motility same as the wild-type Leishmania.
(II) Cultivation for growth (2 weeks)
To further confirm the complete inactivation of Leishmania, we separated 100μl from each sample, [+drug, +light], [+drug, -light] and [-drug;-light], right after illumination. And add them into 24-Well plate containing M199+10%HI-FB medium. Observed and kept tract of it continuously for two weeks. Except the [+drug, +light] group, the other two groups were moving and increasing normally. As for the [+drug, +light] group, there was no motility, or proliferation of the Leishmania from the beginning to the end of the two weeks.
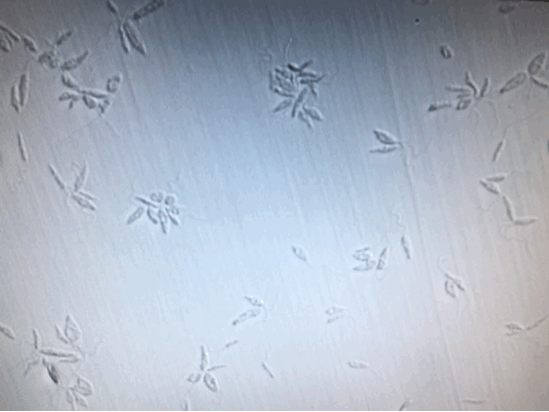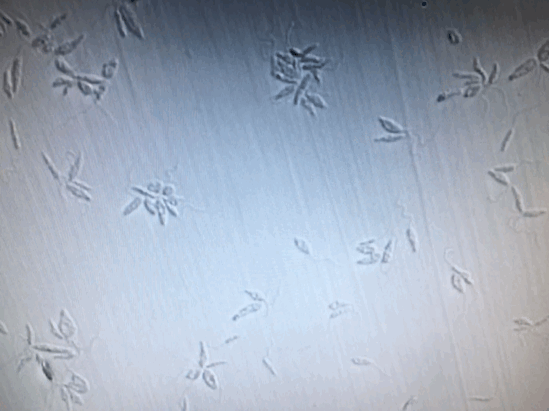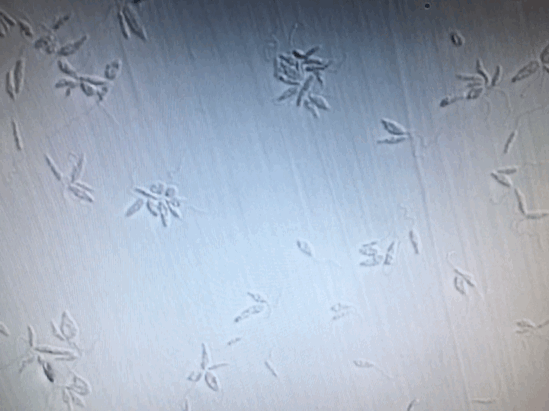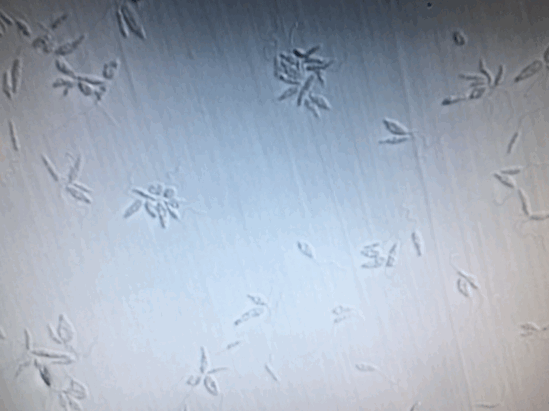


(From left to right: [+drug,+light] [+drug,-light] [-drug,-light])



(From left to right: [+drug,+light] [+drug,-light] [-drug,-light])
(III) 2 months after subcutaneously injecting inactivated Leishmania into mice
We subcutaneously injected mouse with108 of photo-inactivated Leishmania on both sides of the back around the shoulders, and injected normal saline as the control group. There was no skin ulcers or inflammation and no any other Leishmaniasis symptoms among the 10 mouse that serve as our experimental group.
Reference:
1. Dutta, S., et al. (2008). "Transgenic Leishmania model for delta-aminolevulinate-inducible monospecific uroporphyria: cytolytic phototoxicity initiated by singlet oxygen-mediated inactivation of proteins and its ablation by endosomal mobilization of cytosolic uroporphyrin." Eukaryot Cell 7(7): 1146-1157.
2. Chang, K. P., et al. (2016). "New "light" for one-world approach toward safe and effective control of animal diseases and insect vectors from leishmaniac perspectives." Parasit Vectors 9(1): 396.
1. Dutta, S., et al. (2008). "Transgenic Leishmania model for delta-aminolevulinate-inducible monospecific uroporphyria: cytolytic phototoxicity initiated by singlet oxygen-mediated inactivation of proteins and its ablation by endosomal mobilization of cytosolic uroporphyrin." Eukaryot Cell 7(7): 1146-1157.
2. Chang, K. P., et al. (2016). "New "light" for one-world approach toward safe and effective control of animal diseases and insect vectors from leishmaniac perspectives." Parasit Vectors 9(1): 396.
(9) The product safety concern in the future
Environmental concern
Leishmania culture requires a certain osmolarity and combination of nutrients. Therefore, our transgenic Leishmania will not survive as soon as it leaves the medium.
Side effects of adjuvant
There are some potential adverse effects underlying every drug and vaccine. More animal test should be carried out and the most efficient dose in human will be selected before clinical trials. In the clinical study, side effect after immunization will be identified and study design will be modified as well. If our product passes the FDA approval, the report of unexpected effect will be always under inspection.
Leishmania culture requires a certain osmolarity and combination of nutrients. Therefore, our transgenic Leishmania will not survive as soon as it leaves the medium.
Side effects of adjuvant
There are some potential adverse effects underlying every drug and vaccine. More animal test should be carried out and the most efficient dose in human will be selected before clinical trials. In the clinical study, side effect after immunization will be identified and study design will be modified as well. If our product passes the FDA approval, the report of unexpected effect will be always under inspection.
(10) How would your project be used in the real world?
Our Leijuvant can act to facilitate both humeral and cellular immune response which may contribute to the development of vaccine nowadays. Combined with our prediction software, the platform can predict the peptides on MHC molecules and help to optimize the peptide presentation which may benefit to engineer the sequence for further research and developing a more efficient vaccine.
(11)Animal Welfare
All animals were housed in groups of 4–5 per cage in a room maintained at 22 ± 2 °C with 12 h light/ dark cycle (light on at 7:00 a.m. and off at 7:00 p.m.). Food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Chang Gung University.


