| Line 52: | Line 52: | ||
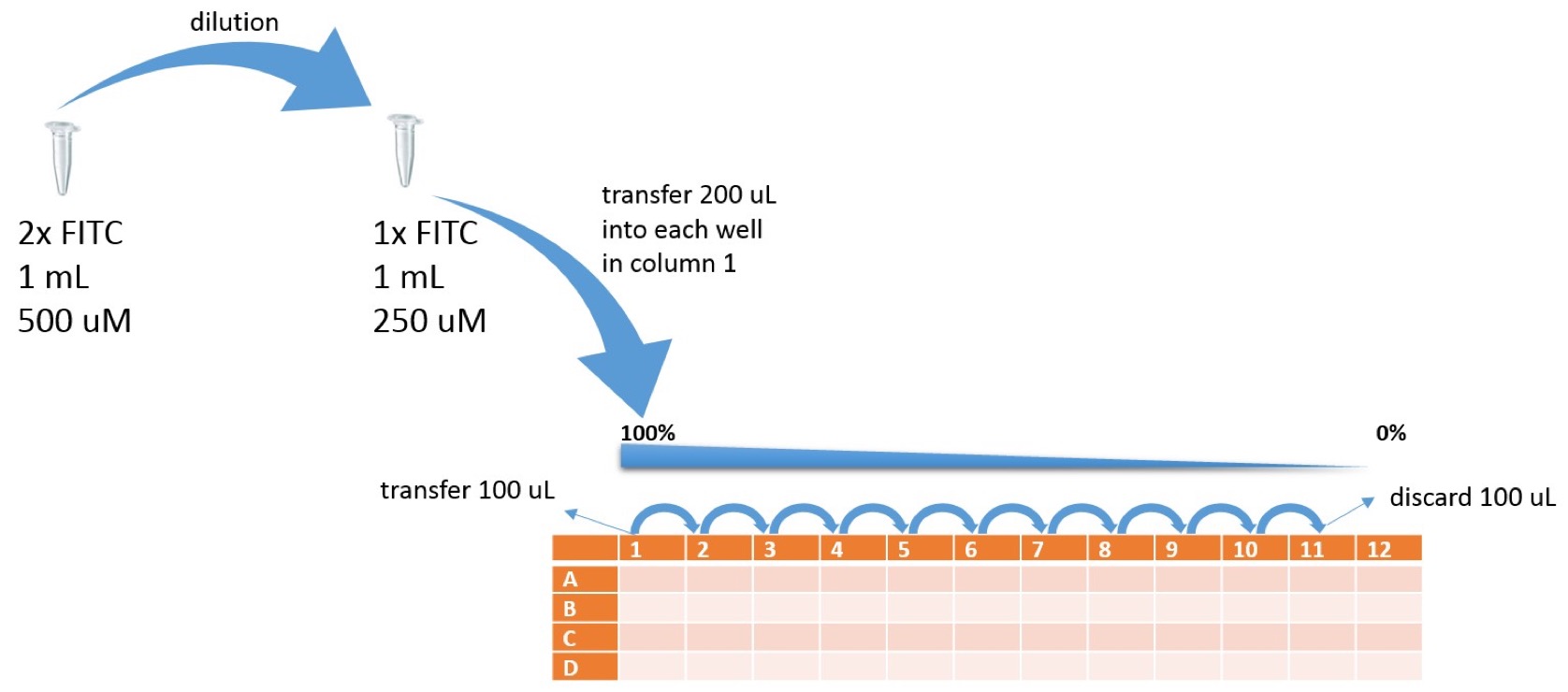
Overview samples in 96 well plate | Overview samples in 96 well plate | ||
| − | + | {{SUSTech_Image_Center_10 | filename=SUSTech_Shenzhen-E3E50BEF-3020-4104-8DAC-538EA7CFFC23.png| caption= | width=1000px }} | |
# Add 100 μl of PBS into wells A2, B2, C2, D2....A12, B12, C12, D12 | # Add 100 μl of PBS into wells A2, B2, C2, D2....A12, B12, C12, D12 | ||
Revision as of 05:51, 19 October 2016

Interlab
Notebook
Contents
Plate Reader Measurement
Calibration
Materials:
1ml LUDOX (provided in kit) ddH2O
96 well plate
Method
1. Add 100 μl LUDOX into wells A1, B1, C1, D1
2. Add 100 μl of H2O into wells A2, B2, C2, D2
3. Measure absorbance 600 nm of all samples in all standard measurement modes in instrument
4. Record the data in the table below or in notebook
5. Import data into Excel (OD600 reference point tab) Sheet_1 provided
Protocol FITC fluorescence standard curve
Materials:
194.7 g FITC (provided in kit)
10ml 1xPBS (phosphate buffered saline) 96 well plate
Method
Prepare the FITC stock solution:
1. Spin down FITC stock tube to make sure pellet is at the bottom of tube.
2. Prepare 2x FITC stock solution (500 μM) by resuspending FITC in 1 mL of 1xPBS
3. Incubate the solution at 42°C for 4 hours
4. Dilute the 2x FITC stock solution in half with 1xPBS to make a 1x FITC solution and resulting concentration of FITC stock solution 250 μM.
Prepare the serial dilutions of FITC:
Overview samples in 96 well plate
- Add 100 μl of PBS into wells A2, B2, C2, D2....A12, B12, C12, D12
- Add 200 μl of FITC 1x stock solution into A1, B1, C1, D1
- Transfer 100 μl of FITC stock solution from A1 into A2.
- Mix A2 by pipetting up and down 3x and transfer 100 μl into A3…
- Mix A3 by pipetting up and down 3x and transfer 100 μl into A4...
- Mix A4 by pipetting up and down 3x and transfer 100 μl into A5...
- Mix A5 by pipetting up and down 3x and transfer 100 μl into A6...
- Mix A6 by pipetting up and down 3x and transfer 100 μl into A7...
- Mix A7 by pipetting up and down 3x and transfer 100 μl into A8...
- Mix A8 by pipetting up and down 3x and transfer 100 μl into A9...
- Mix A9 by pipetting up and down 3x and transfer 100 μl into A10...
- Mix A10 by pipetting up and down 3x and transfer 100 μl into A11...
- Mix A11 by pipetting up and down 3x and transfer 100 μl into liquid waste
- Repeat dilution series for rows B, C, D
- Measure fluorescence of all samples in all standard measurement modes in instrument
- Record the data in notebook
- Import data into Excel (FITC standard curve tab) Sheet_1 provided
Cell measurement protocol
Materials:
Competent cells (Escherichia coli strain DH5α)
LB (Luria Bertani) media with Chloramphenicol (stock concentration 25 mg/mL dissolved in EtOH)
1 ml Falcon tube for cell growth Incubator at 37°C
1.5 ml eppendorf tubes for sample storage Ice bucket with ice
Pipettes
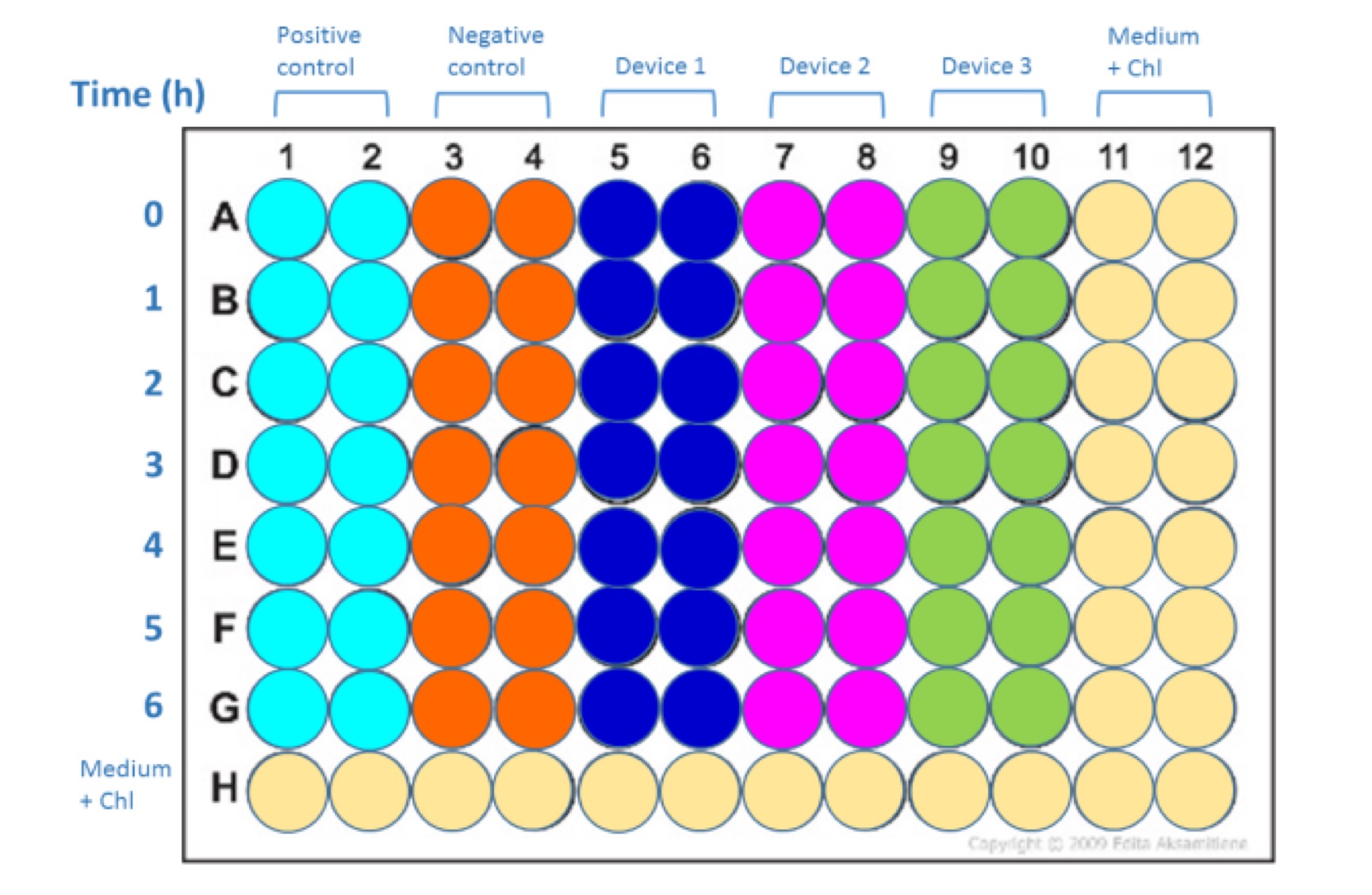
Devices (from InterLab Measurement Kit):
• Positive control
• Negative control
• Device 1: J23101+I13504
• Device 2: J23106+I13504
• Device 3: J23117+I13504
Method
Day 1: transform Escherichia coli DH5α or TOP10 with these following plasmids:
• Positive control
• Negative control
• Device 1: J23101+I13504
• Device 2: J23106+I13504
• Device 3: J23117+I13504
Day 2: Pick 2 colonies from each of plate and inoculate it on 5-10 mL LB medium + Chloramphenicol. Grow the cells overnight (16-18 hours) at 37°C and 220 rpm.
Day 3: Cell growth, sampling, and assay
- Set instrument to read OD600 (as OD calibration setting)
- Measure OD600 of the overnight cultures
- Record data in notebook
- Import data into Excel (normalisation tab) Sheet_1 provided
- Dilute the cultures to a target OD600 of 0.02 (see the volume of preloading culture and media in Excel (normalisation tab) Sheet_1) in 10 ml LB medium with chloramphenicol in 50 mL falcon tube
- Incubate the cult3ures at 37°C and 220 rpm.
- Take 100 µL (1% of total volume) samples of the cultures at 0, 1, 2, 3, 4, 5, and 6 hours of incubation
- Place samples on ice.
- At the end of sampling point need to measure samples (OD and Fl measurement), see the below for details.
- Record data in notebook
- Import data into Excel (cell measurement tab) Sheet_1 provided
Measurement
It is important that use the same instrument settings that used when measuring the FITC standard curve. This includes using the sample volume (100 ul) or 1 mL sample for measurement using spectrophotometer.
Samples should be laid out according to Fig. 2. Pipette 100 µl of each sample into each well. Set the instrument settings as those that gave the best results in calibration curves (no measurements off scale). If necessary can test more than one of the previously calibrated settings to get the best data (no measurements off scale).
Lay out for Abs600 and Fluorescence measurement
Flow Cytometer Measurement
Materials
- 96 well plate
- 194.7 g FITC (provided in kit)
- 10ml 1xPBS (phosphate buffered saline) 96 well plate
- Competent cells (Escherichia coli strain DH5α)
- LB (Luria Bertani) media with Chloramphenicol (stock concentration 25 mg/mL
dissolved in EtOH), 1 ml Falcon tube for cell growth Incubator at 37°C, 1.5ml eppendorf tubes for sample storage Ice bucket with ice,Pipettes, SpheroTech Rainbow Calibration Particles RCP-30-5A, CytoFlex flowcytometer.
Devices (from InterLab Measurement Kit):
• Positive control
• Negative control
• Device 1: J23101+I13504
• Device 2: J23106+I13504
• Device 3: J23117+I13504
Methods
Open computer, click cytometer setting, load clean solution and system startup program for initialization.
Load QC(Lot: 45065) falcon tube to do pre-tests.
Load Rainbow beads, set FSC-A-SSA, FSC-A-FSC-H, FITC-A-Count.
Mix 100ul overnight culture with PBS, load samples and examine the fluorescence.
Close experiment and perform daily clean with ddH2O. Exit the software.