| Line 112: | Line 112: | ||
S_0 + DBxb1 & \rightleftharpoons S_1 \\ | S_0 + DBxb1 & \rightleftharpoons S_1 \\ | ||
S_1 + DBxb1 & \rightleftharpoons S_2 \\ | S_1 + DBxb1 & \rightleftharpoons S_2 \\ | ||
| − | S_2 | + | S_2 & \rightarrow P^{switched} \\ |
mRNA_{int} & \rightarrow \\ | mRNA_{int} & \rightarrow \\ | ||
Bxb1 & \rightarrow \\ | Bxb1 & \rightarrow \\ | ||
Revision as of 11:52, 3 October 2016
SWITCH MODULE
QUICK LINKS
These links will quickly take you to the aspects of our work you may be interested in:
GOALS
- Provide qualitative information for the design of the switch.
- Maximize the ratio between true and false positives.
- Show if the population effect can be used for retrieving quantitative informations.
MODEL
We designed and modeled several variants of the switch.
VERSION 1: BXB1, TP901 AND PHIC31 INTEGRASES

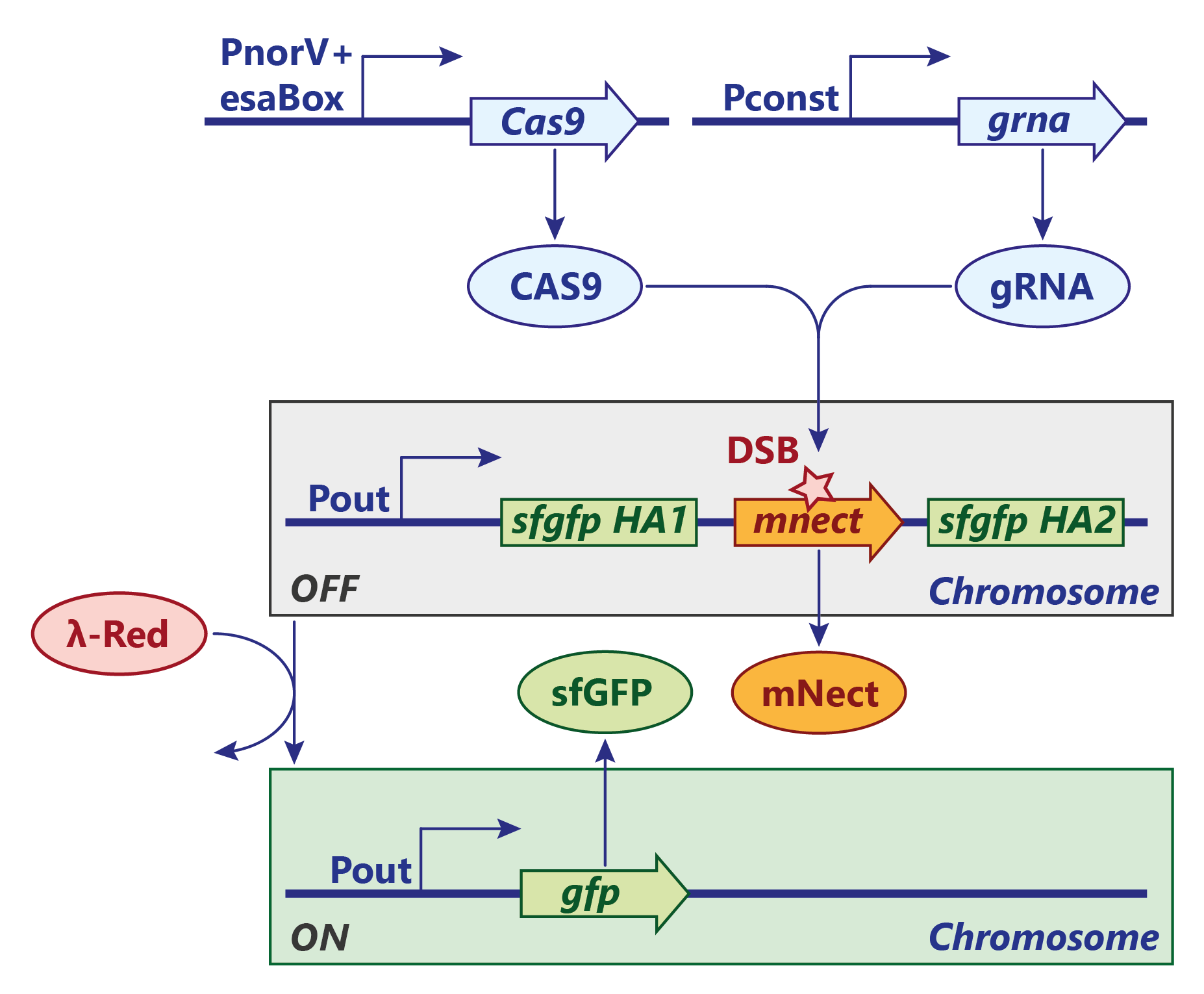
Figure 1: Integrase switch design, the same design applies to Bxb1, Tp901 and PhiC31. Click to enlarge.
The simplest version of the switch is based on the integrase family of recombinases. On this page we focus on the Bxb1 integrase, but the same model and analysis applies to Tp901 and PhiC31.
From the literature1, the dynamics of Bxb1 appear to be well known. When the AND gate in the sensor module activates, Bxb1 is expressed and dimerizes. In its dimerized form, Bxb1 can bind to the attB and attR binding sites placed around the Pout promoter. When both sites are occupied, synapsis between the two can happen, enabling flipping.
The flipping process changes the direction of the reporter promoter and transforms the attB, attP sites in attL, attR. At this point the switch is in the ON state, Bxb1 dimers can still bind to the attL and attR sites, but flipping the sequence again.
The following section describes the species and reactions involved.
REACTIONS
\begin{align*} P_{hyb} & \rightarrow P_{hyb} + mRNA_{int} \\ mRNA_{int} & \rightarrow mRNA_{int} + Bxb1 \\ Bxb1 + Bxb1 & \rightleftharpoons DBxb1 \\ S_0 + DBxb1 & \rightleftharpoons S_1 \\ S_1 + DBxb1 & \rightleftharpoons S_2 \\ S_2 & \rightarrow P^{switched} \\ mRNA_{int} & \rightarrow \\ Bxb1 & \rightarrow \\ DBxb1 & \rightarrow \\ \end{align*}SPECIES
| Name | Description |
|---|---|
| PhybON | Fraction of activity of the AND gate hybrid promoter |
| mRNAint | mRNA of the integrase |
| Bxb1 | Integrase protein |
| DBxb1 | Dimerized form of the integrase protein |
| S0 | Plasmid with free attB and attP sites. |
| S1 | Plasmid with DBxb1 bound to the attB or attP site. |
| S2 | Plasmid with DBxb1 bound to both the attB and attP sites. |
| Pswitched | Plasmid with switched reporter promoter. |
RESULTS
POPULATION EFFECT ANALISYS
TUNING FOR THE TARGET TIMESCALE
In the context of IBD investigation, we know that the signals we want to detect in the gut last for 2 to 6 hours. Thus it is desirable that the system requires the same amount of time to reach full switching.
The parameters that can be easily modified in the biological implementation are the invertase translation rate (RBS engineering) and degradation rate (degradation tags). A senstivity analysis (PLOT) showed that these parameters are indeed sensitive to variations, and their adjustment can make the system work optimally.
PLACEMENT OF THE INTEGRASE GENE
For the integrase version of the switch we evaluated two different designs trying to reduce the effects of the leakiness caused by the AND gate:
- The integrase gene is placed outside the flipping cassette.
- The integrase gene is placed inside the flipping cassette.
In the latter case the expression of the invertase protein decreases when plasmids start to switch. An early qualitative simulation showed that option 2 slightly reduces leakiness, but it also make complete flipping almost impossible. We conclude that option 1 is preferrable. (PLOT)
CHOICE OF PLASMID ORIs