| Line 91: | Line 91: | ||
<img src="https://static.igem.org/mediawiki/2016/d/d6/T--ETH_Zurich--switch_integrase.png"> | <img src="https://static.igem.org/mediawiki/2016/d/d6/T--ETH_Zurich--switch_integrase.png"> | ||
</a> | </a> | ||
| − | <p><b>Figure | + | <p><b>Figure 2:</b> Integrase switch design, the same design applies to Bxb1, Tp901 and PhiC31. Click to enlarge.</p> |
</div> | </div> | ||
<p>The simplest version of the switch is based on the integrase family of recombinases. On this page we focus on the Bxb1 integrase, but the same model and analysis applies to Tp901 and PhiC31.</p> | <p>The simplest version of the switch is based on the integrase family of recombinases. On this page we focus on the Bxb1 integrase, but the same model and analysis applies to Tp901 and PhiC31.</p> | ||
| Line 169: | Line 169: | ||
<img src="https://static.igem.org/mediawiki/2016/3/35/T--ETH_Zurich--switch_crispr_cas9.png"> | <img src="https://static.igem.org/mediawiki/2016/3/35/T--ETH_Zurich--switch_crispr_cas9.png"> | ||
</a> | </a> | ||
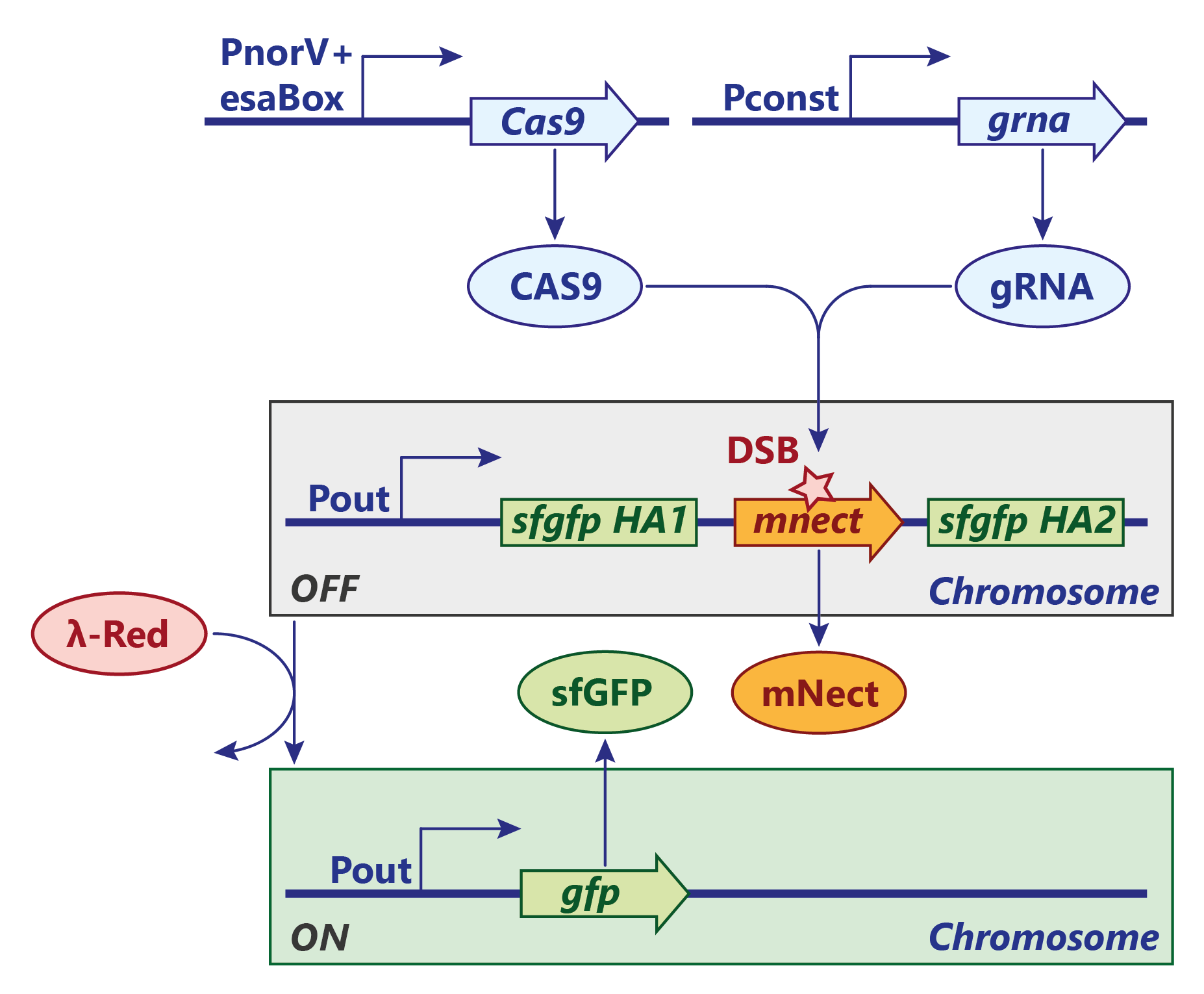
| − | <p><b>Figure | + | <p><b>Figure 3:</b> CRISPR/Cas9 switch design. Click to enlarge.</p> |
</div> | </div> | ||
<p>The first one of our CRISPR switches is based on the Cas9 system.</p> | <p>The first one of our CRISPR switches is based on the Cas9 system.</p> | ||
</div> | </div> | ||
</div> | </div> | ||
| − | <div class="sec white | + | <div class="sec white content_only"> |
<div> | <div> | ||
<h3>VERSION 3: CRISPR/CPF1 CHROMOSOMAL GENE EDITING</h3> | <h3>VERSION 3: CRISPR/CPF1 CHROMOSOMAL GENE EDITING</h3> | ||
| Line 181: | Line 181: | ||
<img src="https://static.igem.org/mediawiki/2016/b/b6/T--ETH_Zurich--switch_crispr_cpf1.png"> | <img src="https://static.igem.org/mediawiki/2016/b/b6/T--ETH_Zurich--switch_crispr_cpf1.png"> | ||
</a> | </a> | ||
| − | <p><b>Figure | + | <p><b>Figure 4:</b> CRISPR/Cpf1 switch design. Click to enlarge.</p> |
</div> | </div> | ||
<p>The last variant is based on CRISPR/Cpf1.</p> | <p>The last variant is based on CRISPR/Cpf1.</p> | ||
| Line 191: | Line 191: | ||
<h2>RESULTS</h2> | <h2>RESULTS</h2> | ||
| − | <h3> | + | <h3>DESIGN IMPROVEMENTS</h3> |
| + | <p>We used the model to suggest important changes to the initial design of the system. In the initial version of the system, the integrase gene was placed <i>inside</i> the flipping cassette. This choice was taken with the intention to reduce the effect of the leakiness: on the plasmids where the cassette with the gene is flipped, the integrase protein is not expressed anymore. We expected cells where leakiness causes partial switching to express less integrase and thus reduce further switching.</p> | ||
| + | |||
| + | <p>However, simulations of the system showed that this approach indeed reduces switching under leakiness conditions, but it also makes more difficult to reach complete switching when the AND gate is fully active. We proposed a different design in which the integrase gene and the flipping cassette are placed on different plasmids and compares its behavior with the initial design.</p> | ||
| + | |||
| + | <div class="image_box full_size"> | ||
| + | <a href="https://2016.igem.org/File:T--ETH_Zurich--switch_comparison.png"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/4/43/T--ETH_Zurich--switch_comparison.png"> | ||
| + | </a> | ||
| + | <p><b>Figure 5:</b> Comparison of the behavior of two designs of the switch in leakiness and activation conditions. The new design is clearly more suitable for our system.</p> | ||
| + | </div> | ||
| + | |||
| + | <p>Figure 5 shows the differences in the qualitative bahavior. The plots qualitatively describe the percentage of flipped cassettes over 6 hours. The simulation proves that the new design is slighlty more sensible to leakiness (0.2% over 6 hours) but takes the percentage of switched cassettes from 80% to 100%. Since 20% is an important difference in the signal, we prefer discarded the initial design and implemented the new one.</p> | ||
<h3>TUNING FOR THE TARGET TIMESCALE</h3> | <h3>TUNING FOR THE TARGET TIMESCALE</h3> | ||
| − | <p>In the context of IBD investigation, we | + | <p>In the context of IBD investigation, we have specific requirements for our system. We propose two different sets of requirements, that allow two different interpretations of the output: binary or quantitative.</p> |
| − | + | ||
| − | < | + | <h4>BINARY OUTPUT</h4> |
| − | + | In this version the circuit must respond to the simple question: "Was a significant concentration of target biomarker present in the gut during inflammation?". | |
| − | + | ||
| − | + | <p>The parameters that can be easily modified in the biological implementation are the invertase translation rate (RBS engineering) and degradation rate (degradation tags). A senstivity analysis (PLOT) showed that these parameters are indeed sensitive to variations, and their adjustment can make the system work optimally.</p> | |
| − | + | ||
| − | + | ||
| − | <p> | + | |
<h3>CHOICE OF PLASMID ORIs</h3> | <h3>CHOICE OF PLASMID ORIs</h3> | ||
| Line 218: | Line 226: | ||
</body> | </body> | ||
</html> | </html> | ||
| + | |||
{{:Template:ETH_Zurich/footer}} | {{:Template:ETH_Zurich/footer}} | ||
Revision as of 08:19, 10 October 2016
SWITCH MODULE
OVERVIEW
The switch is the memory element of our circuit. We designed and tested different variants, all of them are based on editing of the plasmids (integrase-based switches) or of the chromosome (CRISPR-based switches).
In general switches are binary elements. However, in biological systems switching doesn't happen at the same time on different plasmids and in different cells. We exploit this property to obtain quantitative outputs by relating the measured fluorescence to the exposure time/concentration of the sensed molecules.
We use the model of the switch to describe the relationship between the amount of switched elements in our cell population and the sensed input. Moreover the model suggested us an improvement on the original design and helped us tuning the switch for the timescale of the events we want to detect in the gut.
GOALS
- Provide a proof that the switch can work as expected.
- Assist the design of the switch.
- Optimize the parameters for the requirements of our application.
- Characterize the kinetics of the invertases precisely.
MODEL
We designed and modeled several variants of the switch. The first design is based on the integrase family of recombinases (Bxb1, Tp901, PhiC31). As an alternative we designed two CRISPR-based switches (CRISPR/Cas9, CRISPR/Cpf1).
VERSION 1: BXB1, TP901 AND PHIC31 INTEGRASES

Figure 2: Integrase switch design, the same design applies to Bxb1, Tp901 and PhiC31. Click to enlarge.
The simplest version of the switch is based on the integrase family of recombinases. On this page we focus on the Bxb1 integrase, but the same model and analysis applies to Tp901 and PhiC31.
From the literature1, the dynamics of Bxb1 appear to be well known. When the AND gate in the sensor module activates, Bxb1 is expressed and dimerizes. In its dimerized form, Bxb1 can bind to the attB and attR binding sites placed around the Pout promoter. When both sites are occupied, synapsis between the two can happen, enabling flipping.
The flipping process changes the direction of the reporter promoter and transforms the attB, attP sites in attL, attR. At this point the switch is in the ON state, Bxb1 dimers can still bind to the attL and attR sites, but flipping the sequence again.
The following section describes the species and reactions involved.
REACTIONS
\begin{align*} P_{hyb} & \rightarrow P_{hyb} + mRNA_{int} \\ mRNA_{int} & \rightarrow mRNA_{int} + Bxb1 \\ Bxb1 + Bxb1 & \rightleftharpoons DBxb1 \\ S_0 + DBxb1 & \rightleftharpoons S_1 \\ S_1 + DBxb1 & \rightleftharpoons S_2 \\ S_2 & \rightarrow P^{switched} \\ mRNA_{int} & \rightarrow \\ Bxb1 & \rightarrow \\ DBxb1 & \rightarrow \\ \end{align*}SPECIES
| Name | Description |
|---|---|
| PhybON | Fraction of activity of the AND gate hybrid promoter |
| mRNAint | mRNA of the integrase |
| Bxb1 | Integrase protein |
| DBxb1 | Dimerized form of the integrase protein |
| S0 | Plasmid with free attB and attP sites. |
| S1 | Plasmid with DBxb1 bound to the attB or attP site. |
| S2 | Plasmid with DBxb1 bound to both the attB and attP sites. |
| Pswitched | Plasmid with switched reporter promoter. |
RESULTS
DESIGN IMPROVEMENTS
We used the model to suggest important changes to the initial design of the system. In the initial version of the system, the integrase gene was placed inside the flipping cassette. This choice was taken with the intention to reduce the effect of the leakiness: on the plasmids where the cassette with the gene is flipped, the integrase protein is not expressed anymore. We expected cells where leakiness causes partial switching to express less integrase and thus reduce further switching.
However, simulations of the system showed that this approach indeed reduces switching under leakiness conditions, but it also makes more difficult to reach complete switching when the AND gate is fully active. We proposed a different design in which the integrase gene and the flipping cassette are placed on different plasmids and compares its behavior with the initial design.

Figure 5: Comparison of the behavior of two designs of the switch in leakiness and activation conditions. The new design is clearly more suitable for our system.
Figure 5 shows the differences in the qualitative bahavior. The plots qualitatively describe the percentage of flipped cassettes over 6 hours. The simulation proves that the new design is slighlty more sensible to leakiness (0.2% over 6 hours) but takes the percentage of switched cassettes from 80% to 100%. Since 20% is an important difference in the signal, we prefer discarded the initial design and implemented the new one.
TUNING FOR THE TARGET TIMESCALE
In the context of IBD investigation, we have specific requirements for our system. We propose two different sets of requirements, that allow two different interpretations of the output: binary or quantitative.
BINARY OUTPUT
In this version the circuit must respond to the simple question: "Was a significant concentration of target biomarker present in the gut during inflammation?".The parameters that can be easily modified in the biological implementation are the invertase translation rate (RBS engineering) and degradation rate (degradation tags). A senstivity analysis (PLOT) showed that these parameters are indeed sensitive to variations, and their adjustment can make the system work optimally.
CHOICE OF PLASMID ORIs





 GOALS
GOALS