| Line 413: | Line 413: | ||
==== Result: ==== | ==== Result: ==== | ||
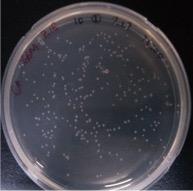
| − | {{ | + | {{SUSTech_Image_Left | filename=SUSTech Shenzhen-C89646A9-AD79-4481-95BB-E54BBC7C1B87.png | caption=Figure 1. Water control | width=200px }} |
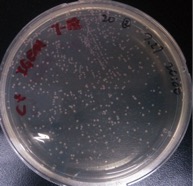
{{SUSTech_Image_Left | filename=SUSTech_Shenzhen-D6D86280-E0BE-43CE-A096-8C835F4D8426.png | caption=Figure 2. 0.5 pg/ul | width=200px }} | {{SUSTech_Image_Left | filename=SUSTech_Shenzhen-D6D86280-E0BE-43CE-A096-8C835F4D8426.png | caption=Figure 2. 0.5 pg/ul | width=200px }} | ||
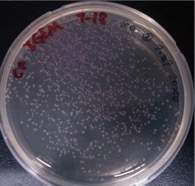
{{SUSTech_Image_Left | filename=SUSTech_Shenzhen-9A343E4A-0565-47D6-BD89-A9FA00F5EEA2.png | caption=Figure 3. 5 pg/ul | width=200px }} | {{SUSTech_Image_Left | filename=SUSTech_Shenzhen-9A343E4A-0565-47D6-BD89-A9FA00F5EEA2.png | caption=Figure 3. 5 pg/ul | width=200px }} | ||
Latest revision as of 06:06, 19 October 2016

Biobricks
Notebook
Contents
- 1 8 Chromoprotein
- 1.1 Plasmid Construction
- 1.2 7.22
- 1.3 7.23
- 1.4 7.24
- 1.5 7.25
- 1.6 7.26
- 1.7 7.27
- 1.8 7.28
- 1.9 7.29
- 1.10 7.30
- 1.10.1 Picking single colonies of ⑧, ⑩, 11
- 1.10.2 Go Gel Electrophoresis and Gel Extraction of ⑤,⑥,⑦,⑧,⑨
- 1.10.3 Enzyme Digestion for Gel Extraction of ⑤,⑥,⑦,⑨ (Redo)
- 1.10.4 Plasmid extraction of ⑧,⑩,11
- 1.10.5 Enzyme Digestion for Gel Extraction of ⑧,⑩,11
- 1.10.6 Go Gel Electrophoresis and Gel Extraction of ⑤,⑥,⑦,⑨ (Redo)
- 1.11 7.31
- 1.12 8.1
- 1.13 8.2
- 1.14 8.3
- 1.15 8.4
- 1.16 8.5
- 1.17 8.6
- 1.18 8.7
- 1.18.1 Enzyme Digestion and Go Gel Electrophoresis Test of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤ C and D
- 1.18.2 Observation of LB medium color of ⑩+⑥+⑤, ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑦+⑤, 11+⑧+⑤, 11+⑨+⑤
- 1.18.3 Picking single colonies of ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑧+⑤, 11+⑨+⑤ (Because there are obvious correct color colonies on those plates)
- 1.18.4 Ligation of ⑩ and ⑥+⑤, 11 and ⑦+⑤
- 1.18.5 Plasmid extraction of ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑧+⑤, 11+⑨+⑤
- 1.19 8.9
- 2 sfGFG
- 2.1 8.2
- 2.2 8.3
- 2.3 8.16
- 2.4 8.17
- 2.5 8.18
- 2.6 8.18
- 2.7 9.21
- 2.8 9.22
- 2.9 9.23
- 2.10 9.24
- 3 Eukaryon Antibiotic
- 3.1 8.31
- 3.2 9.2
- 3.3 9.3
- 3.4 9.4
- 3.5 9.12
- 3.6 9.13
- 3.7 9.14
- 3.8 9.15
- 3.9 10.5
- 3.10 10.6
- 3.11 10.7
- 4 GECO
- 4.1 9.23
- 4.2 9.24
- 4.3 9.25
- 4.4 9.26
- 4.5 9.27
- 4.6 9.28
- 4.7 9.29
- 4.8 =Enzyme Digestion and Go Gel Electrophoresis Test of M2GECO
- 5 TetOn
8 Chromoprotein
Plasmid Construction
ALL ABBREVIATIONS USED:
| Name | Number | Index | Position | Backbone | Length |
|---|---|---|---|---|---|
| Strong promoter | ① | BBa_J23100 | 4-17-D | BBa_J61002 | 35 |
| Weak promoter | ② | BBa_J23106 | 4-17-P | BBa_J61002 | 35 |
| Strong RBS | ③ | BBa_B0034 | 4-1-N | pSB1A2 | 12 |
| Weak RBS | ④ | BBa_B0031 | 2-2-H | pSB1C3 | 14 |
| Terminator | ⑤ | BBa_B0015 | 3-3-F | pSB1C3 | 129 |
| eforRed | ⑥ | BBa_K592012 | 6-15-I | pSB1C3 | 681 |
| gfasPurple | ⑦ | BBa_K1033919 | 6-9-K | pSB1C3 | 669 |
| FwYellow | ⑧ | BBa_K1033910 | 4-6-K | pSB1C3 | 714 |
| SpisPink | ⑨ | BBa_K1033932 | 6-11-k | pSB1C3 | 678 |
| ①+③ | ⑩ | BBa_K880005 | 2-3-F | pSB1C3 | 55 |
| Medium promoter+④ | 11 | BBa_K608007 | 1-5-G | pSB1C3 | 57 |
7.22
Transformation of ①, ③, ⑤
Time: 20:30
Handler: Liao Weiduo
Procedure: IGEM Protocols
1. Thaw competent cells on ice.
2. Punch a hole into the corresponding hole in the hole in 2016 kit plate by a 10 ul pipette tip. Adding 10ul ddH2O to the hole and pipette up and down for several times. Let sit for several minutes to make sure the dyed DNA is fully resuspended.
3. Pipette 50ul of competent cells into 1.5ml tube.
4. Pipette 1ul of resuspended DNA into 1.5ml tube.
5. Pipette 1ul of ddH2O as a control into 1.5ml tube.
6. Close 1.5ml tubes, incubate on ice for 30 min.
7. Heat shock tubes at 42℃ for 1 min.
8. Incubate on ice for 5 min.
9. Pipette 200ul SOC media to each transformation.
10. Incubate at 37℃ for 2 hours, 220rpm.
11. Pipette each transformation on petri plates for a 100ul, and spread with sterilized spreader immediately.
12. Incubate transformations overnight at 37℃.
7.23
Picking single colonies of ①, ③, ⑤
Time: 16:00
Handler: Liao Weiduo
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics, each plate we pick 2 single colonies, A and B.
- Incubate overnight at 37℃, 220rpm.
7.24
Plasmid extraction of ①, ③, ⑤ A and B
Time: 9:00
Handler: Liao Weiduo
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
- Add 2ml LB medium to a 2ml microcentrifuge tube.
- Centrifuge at 10,000 g for 1 minute at roonm temperature.
- Discard the supernatant.
- Repeat Step1-3 until 4ml sample has been transferred to the 2ml tube.
- Add 250ul Solution I/RNase A. Vortex to mix thoroughly.
- Add 250ul Solution II. Invert and gently rotate the tube several times to obtain a clear lysate. A 2-3 minute incubation may be necessary.
- Add 350ul Solution III. Immediately invert several times until a flocculent white precipitate forms.
- Centrifuge at maximum speed (>13,000 g) for 10 minutes.
- Insert a HiBind DNA Mini Column into a 2ml Collection Tube.
- Transfer the cleared supernatant (~830ul)from Step 8 by CAREFULLY aspirating it into the HiBind DNA Mini Column.
- Centrifuge at maximum speed for 1 minute.
- Discard the filtrate and reuse the collection tube.
- Add 500ul HBC Buffer. (HBC Buffer must be diluted with isopropanol before use)
- Centrifuge at maximum speed for 1 min.
- Discard the filtrate and reuse collection tube.
- Add 700ul DNA Wash Buffer. (DNA Wash Buffer must be diluted with 100% ethanol prior to use.)
- Centrifuge at maximum speed for 1 min.
- Discard the filtrate and reuse the collection tube.
- Repeat Steps 16-18.
- Centrifuge the empty HiBind DNA Mini Column for 2 minutes at maximum speed to dry the column matrix.
- Transfer the HiBind DNA Mini Column to a clean 1.5ml microcentrifuge tube.
- Add 80ul ddH2O.
- Let sit at room temperature for 2 min.
- Centrifuge at maximum speed for 1 min.
Results:
‘’’Concentration:’’’ ①A: 245.9 ng/ul 1.84; ①B: 340.5 ng/ul 1.84; ③A: 116.2 ng/ul 1.65; ③B: 102.1 ng/ul 1.77; ⑤A: 151.4 ng/ul 1.80; ⑤B: 68.0 ng/ul 1.84;
7.25
Transformation of ②,④,⑥,⑦,⑧,⑨
Time: 0:00
Handler: Tang Shiqiang
Procedure: IGEM Protocols
Picking single colonies of ②,④,⑥,⑦,⑧,⑨
Time: 20:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics, each plate we pick 2 single colonies, α and β.
- Incubate overnight at 37℃, 220rpm.
7.26
Plasmid extraction of ②,④,⑥,⑦,⑧,⑨ group α
Time: 9:40
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ②α: 369.8 ng/ul; ④α: 142.4 ng/ul; ⑥α: 116.5 ng/ul; ⑦α: 92 ng/ul; ⑧α: 157.4 ng/ul; ⑨α: 168.3 ng/ul.
‘’’ Note: ’’’ ⑧ has a red colony.
Plasmid extraction of ②,④,⑥,⑦,⑧,⑨ group β
Time: 14:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ②β: 496.9 ng/ul 1.86; ④β: 256.4 ng/ul 1.85; ⑥β: 220.3 ng/ul 1.85; ⑦β: 225.7 ng/ul 1.85; ⑧β: 304.6 ng/ul 1.85; ⑨β: 352.2 ng/ul 1.84.
‘’’ Note: ‘’’ ⑧ has a red colony.
7.27
Competent Cell Test
Time: 16:00
Handler: Lu Shixin
Procedure: IGEM Protocols
- Spin down the DNA tubes form the Competent Cell Test Kit to collect all of the DNA into the bottom of each tube prior to use.
- Thaw competent cells on ice.
- Pipet 50ul of competent cells into each tube.
- Pipet 1ul of DNA into each microcentrifuge tube.
- Incubate on ice for 30 min.
- Heat shock at 42℃ for 1 min.
- Incubate on ice for 5 min.
- Add 200ul SOC media, incubate at 37℃ for 2h.
- Pipet 20ul from each tube onto the appropriate plate, and spread it.
- Incubate at 37℃ overnight, approximately 16 h.
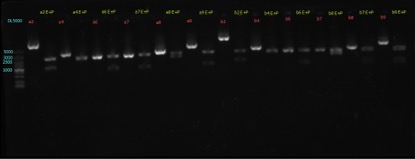
Enzyme Digestion and Go Gel Electrophoresis Test of ②,④,⑥,⑦,⑧,⑨
Time: 20:00
Handler: Xiao Tianyao
Procedure:
1. Make enzyme cutting system solution.
| ②α | ④α | ⑥α | ⑦α | ⑧α | ⑨α | |
|---|---|---|---|---|---|---|
| DNA(400ng) | 1ul | 2ul | 2ul | 2ul | 1.2ul | 1.2ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| EcoRI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| PstI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 16.6ul | 15.6ul | 15.6ul | 15.6ul | 16.4ul | 16.4ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
| ②β | ④β | ⑥β | ⑦β | ⑧β | ⑨β | |
|---|---|---|---|---|---|---|
| DNA(400ng) | 1ul | 2ul | 2ul | 2ul | 1.2ul | 1.2ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| EcoRI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| PstI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 16.6ul | 15.6ul | 15.6ul | 15.6ul | 16.4ul | 16.4ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
Incubate at 37℃ for 30 min.
Make 1% agarose gel 30ml.
Add 6x loading dye to the enzyme cutting system solution.
Go gel electrophoresis.
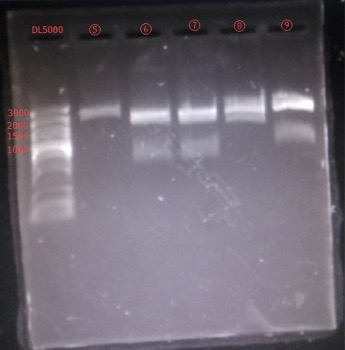
Results:
figures show below:
7.28
Competent Cell Test Colonies Counting
Time: 13:00
Handler: Lu Shixin
Result:
{{SUSTech_Image_Center_10 | filename=SUSTech_Shenzhen-372E5718-44FE-463A-86A8-57C609A8F345.png | caption= ]] And all the colonies are red under UV light.7.29
Transformation of ⑧,⑩,11
Time: 16:00
Handler: Lu Shixin, Tang Shiqiang
Procedure: IGEM Transformation Protocol
Enzyme Digestion for Gel Extraction of ⑤,⑥,⑦,⑧,⑨
Time: 20:00
Handler: LuShixin, Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| ⑥ | ⑦ | ⑧ | ⑨ | |
|---|---|---|---|---|
| DNA(500ng) | 2.5ul | 2.5ul | 2.0ul | 1.5ul |
| Cutsmart buffer | 2.5ul | 2.5ul | 2.5ul | 2.5ul |
| EcoRI-HF | 0.5ul | 0.5ul | 0.5ul | 0.5ul |
| SpeI-HF | 0.5ul | 0.5ul | 0.5ul | 0.5ul |
| ddH2O | 19ul | 19ul | 19.5ul | 20ul |
| Total | 25ul | 25ul | 25ul | 25ul |
| ⑤ | |
|---|---|
| DNA (500ng) | 8ul |
| Cutsmart buffer | 2.5ul |
| EcoRI-HF | 0.5ul |
| XbaI | 0.5ul |
| ddH2O | 16.5ul |
| Total | 25ul |
- Incubate at 37℃ overnight. (Start from 21:20)
7.30
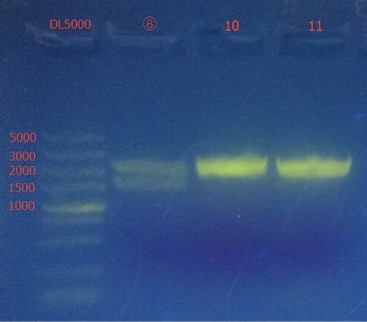
Picking single colonies of ⑧, ⑩, 11
Time: 9:00
Handler: Lu Shixin
Procedure:
- Pick single colonies, adding 1ml LB with corresponding antibiotics in 1.5ml EP tube, each plate we pick 2 single colonies, α and β.
- Incubate for 2h at 37℃.
- Transfer 1ml LB from 1.5ml EP tube to 14ml bacteria shaking tube, and add 5ml corresponding antibiotics LB.
- Incubate at 37℃ for 10h, 220rpm.
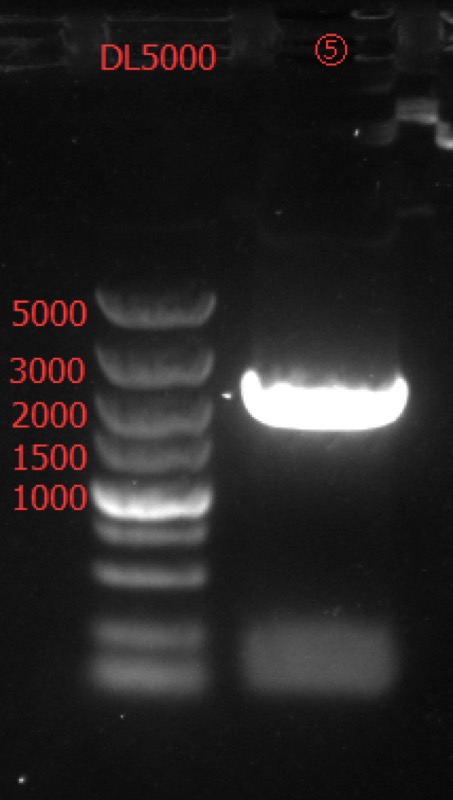
Go Gel Electrophoresis and Gel Extraction of ⑤,⑥,⑦,⑧,⑨
Time: 9:00
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
- Excise the DNA fragment of interest.
- Determine the volume of the gel slice by weighing it in a clean 1.5ml EP tube.
- Add 1 volume Binding Buffer (XP2).
- Incubate at 50-60℃ for 7 min or until the gel has completely melted. Vortex or shake the tube every 2-3 min.
- Insert a HiBind DNA Mini Column in a 2ml Collection Tube.
- Add no more than 700ul DNA/agarose solution from Step 7 to the HiBind DNA Mini Column.
- Centrifuge at 10,000 g for 1 min at room temperature.
- Discard the filtrate and reuse collection tube.
- Repeat Steps 9-11 until all of the sample has been transferred to the column.
- Add 300ul Binding Buffer(XP2).
- Centrifuge at maximum speed (>13,000 g) for 1 min at room temperature.
- Discard the filtrate and reuse collection tube.
- Add 700ul SPW Wash Buffer. (SPW Wash Buffer must be diluted with 100% ethanol prior to use.)
- Centrifuge at maximum speed for 1 min at room temperature.
- Discard the filtrate and reuse collection tube.
- Repeat Steps 16-18.
- Centrifuge the empty HiBind DNA Mini Column for 2 min at maximum speed to dry the column matrix.
- Transfer the HiBind DNA Mini Column to a clean 1.5ml EP tube.
- Add 15-30ul ddH2O.
- Let sit at room temperature for 2 min.
- Centrifuge at maximum speed for 1 min.
Results:
All the concentration of gel extraction are below 10 ng/ul. So we decide to repeat enzyme digestion with more DNA.
Enzyme Digestion for Gel Extraction of ⑤,⑥,⑦,⑨ (Redo)
Time: 18:00
Handler: Lu Shixin
Procedure:
- Make enzyme cutting system solution.
| ⑥ | ⑦ | ⑨ | |
|---|---|---|---|
| DNA(>4ug) | 21.5ul | 21.5ul | 21.5ul |
| Cutsmart buffer | 2.5ul | 2.5ul | 2.5ul |
| EcoRI-HF | 0.5ul | 0.5ul | 0.5ul |
| SpeI-HF | 0.5ul | 0.5ul | 0.5ul |
| ddH2O | 0ul | 0ul | 0ul |
| Total | 25ul | 25ul | 25ul |
| ⑤ | |
|---|---|
| DNA (2ug) | 21.5ul |
| Cutsmart buffer | 2.5ul |
| EcoRI-HF | 0.5ul |
| XbaI | 0.5ul |
| ddH2O | 0ul |
| Total | 25ul |
- Incubate at 37℃ for 3h. (Start from 18:40)
Plasmid extraction of ⑧,⑩,11
Time: 21:00
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
The length of them are correct.
Concentration: ⑧: 130.2 ng/ul; ⑩: 147.0 ng/ul; 11: 93.3 ng/ul.
Enzyme Digestion for Gel Extraction of ⑧,⑩,11
Time: 22:30
Handler: Lu Shixin
Procedure:
- Make enzyme cutting system solution.
| ⑩ | 11 | |
|---|---|---|
| DNA(>1.5ug) | 11.5ul | 21.5ul |
| Cutsmart buffer | 2.5ul | 2.5ul |
| SpeI-HF | 0.5ul | 0.5ul |
| PstI-HF | 0.5ul | 0.5ul |
| ddH2O | 10ul | 0ul |
| Total | 25ul | 25ul |
| ⑧ | |
|---|---|
| DNA (0.5ug) | 4ul |
| Cutsmart buffer | 2.5ul |
| EcoRI-HF | 0.5ul |
| XbaI | 0.5ul |
| ddH2O | 17.5ul |
| Total | 25ul |
- Incubate at 37℃ overnight. (Start from 22:40)
Go Gel Electrophoresis and Gel Extraction of ⑤,⑥,⑦,⑨ (Redo)
Time: 22:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Results:
Gel extraction concentration: ⑤: 15.3 ng/ul; ⑥: 36.3 ng/ul; ⑦: 36.8 ng/ul; ⑨: 26.9 ng/ul.
7.31
Ligation of ⑥+⑤, ⑦+⑤, ⑨+⑤
Time: 0:00
Handler: Tang Shiqiang
Procedure:
- Make ligation system solution.
| ⑥+⑤ | ⑦+⑤ | ⑨+⑤ | |
|---|---|---|---|
| Vector (50ng) | 3.3ul | 3.3ul | 3.3ul |
| Insert | 1.4ul | 1.4ul | 1.9ul |
| ddH2O | 5.3ul | 5.3ul | 4.8ul |
| 2x Quick ligase buffer | 10ul | 10ul | 10ul |
| Quick ligase | 1ul | 1ul | 1ul |
| Total | 21ul | 21ul | 21ul |
- Incubate at room temperature for 5 min.
- Take 2.5ul ligation system solution to do transformation.
Go Gel Electrophoresis and Gel Extraction of ⑧, ⑩, 11
Time: 15:30
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Results:
Gel extraction concentration: ⑩: 67.3 ng/ul 1.65; 11: 66.4 ng/ul 1.69.
‘’‘Note:’‘’ We found that we take a wrong ⑧ before.
Transformation of ⑧
Time: 16:30
Handler: Tang Shiqiang
Procedure: IGEM Transformation Protocol
Picking single colonies of ⑥+⑤, ⑦+⑤, ⑨+⑤
Time: 19:30
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics, each plate we pick 2 single colonies, A and B.
- Incubate overnight at 37℃, 220rpm.
8.1
Picking single colonies of ⑧
Time: 9:30
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics, each plate we pick 2 single colonies, A and B.
- Incubate at 37℃ for about 14h, 220rpm.
Plasmid extraction of ⑥+⑤, ⑦+⑤, ⑨+⑤ A and B
Time: 9:30
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ⑥+⑤A: 126.3 ng/ul 1.86; ⑥+⑤B: 124.9 ng/ul 1.82; ⑦+⑤A: 116.5 ng/ul 1.87; ⑦+⑤B: 112.0 ng/ul 1.86; ⑨+⑤A: 120.4 ng/ul 1.87; ⑨+⑤B: 149.0 ng/ul 1.87;
Enzyme Digestion and Go Gel Electrophoresis Test of ⑥+⑤, ⑦+⑤, ⑨+⑤ A and B
Time: 12:00
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| ⑥+⑤A | ⑦+⑤A | ⑨+⑤A | ⑥+⑤B | ⑦+⑤B | ⑨+⑤B | |
|---|---|---|---|---|---|---|
| DNA(200ng) | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| EcoRI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| PstI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 15.6ul | 15.6ul | 15.6ul | 15.6ul | 15.6ul | 15.6ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
- Incubate at 37℃ for 30 min.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
‘’‘Comments:’‘’ The length of them are incorrect. Their length are larger than 1000bp while should be smaller than 1000bp.
‘’‘Note:’‘’ Finally we found that we used a tube of wrong ⑤.
Put ⑧ A and B at 4℃
Time: 20:00
Handler: Tang Shiqiang
8.2
Plasmid extraction of ⑧ A and B
Time: 14:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ⑧A: 116.5 ng/ul 1.82; ⑧B: 73.2 ng/ul 1.81.
Enzyme Digestion for Gel Extraction of ⑤, ⑧
Time: 22:00
Handler: Lu Shixin, Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| ⑤3-3-F 1 | |
|---|---|
| DNA(>3ug) | 21.5ul |
| Cutsmart buffer | 2.5ul |
| EcoRI-HF | 0.5ul |
| XbaI | 0.5ul |
| ddH2O | 0ul |
| Total | 25ul |
| ⑧A | |
|---|---|
| DNA (>2ug) | 21.5ul |
| Cutsmart buffer | 2.5ul |
| EcoRI-HF | 0.5ul |
| SpeI-HF | 0.5ul |
| ddH2O | 0ul |
| Total | 25ul |
- Incubate at 37℃ overnight. (Start from 22:25)
8.3
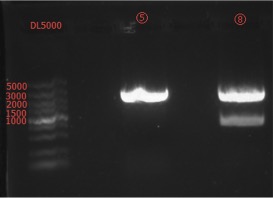
Go Gel Electrophoresis and Gel Extraction of ⑤, ⑧
Time: 10:00
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Results:
Concentration: ⑤: 51.4 ng/ul 1.75; ⑧: 23.2 ng/ul 1.71.
Ligation of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤
Time: 16:30
Handler: Lu Shixin
Procedure:
- Make ligation system solution.
| ⑥+⑤ | ⑦+⑤ | ⑧+⑤ | ⑨+⑤ | |
|---|---|---|---|---|
| Vector (50ng) | 1ul | 1ul | 1ul | 1ul |
| Insert | 1.5ul | 1.5ul | 2.5ul | 2ul |
| ddH2O | 7.5ul | 7.5ul | 6.5ul | 7ul |
| 2x Quick ligase buffer | 10ul | 10ul | 10ul | 10ul |
| Quick ligase | 1ul | 1ul | 1ul | 1ul |
| Total | 21ul | 21ul | 21ul | 21ul |
- Incubate at room temperature for 10 min.
- Take 2ul ligation system solution to do transformation.
8.4
Picking single colonies of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤
Time: 9:30
Handler: Lu Shixin
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics, each plate we pick 2 single colonies, A and B.
- Incubate at 37℃ for about 12h, 220rpm.
Plasmid extraction of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤ A and B
Time: 21:30
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ⑥+⑤A: 54.4 ng/ul 2.18; ⑥+⑤B: 29.2 ng/ul 3.15; ⑦+⑤A: 55.4 ng/ul 2.42; ⑦+⑤B: 58.8 ng/ul 2.42; ⑧+⑤A: 70.0 ng/ul 2.22; ⑧+⑤B: 35.6 ng/ul 2.84; ⑨+⑤A: 65.9 ng/ul 2.23; ⑨+⑤B: 74.3 ng/ul 2.18;
‘’’Comments:’’’ All the concentration seems to have some problem, and the A260/280 have some problem.
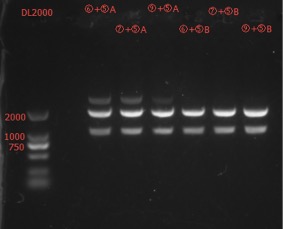
Enzyme Digestion and Go Gel Electrophoresis Test of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤ A and B
Time: 23:00
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| ⑥+⑤A | ⑦+⑤A | ⑧+⑤A | ⑨+⑤A | ⑥+⑤B | ⑦+⑤B | ⑧+⑤B | ⑨+⑤B | |
|---|---|---|---|---|---|---|---|---|
| DNA(200ng) | 4ul | 4ul | 3ul | 3ul | 7ul | 4ul | 7ul | 3ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| XbaI | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| PstI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 13.6ul | 13.6ul | 14.6ul | 14.6ul | 10.6ul | 13.6ul | 10.6ul | 14.6ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
- Incubate at 37℃ for 30 min.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
The length seems to be correct.
8.5
Enzyme Digestion for Gel Extraction of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤
Time: 2:20
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| ⑥+⑤A | ⑦+⑤B | ⑧+⑤A | ⑨+⑤B | |
|---|---|---|---|---|
| DNA(>1.5ug) | 26ul | 26ul | 26ul | 26ul |
| Cutsmart buffer | 3ul | 3ul | 3ul | 3ul |
| EcoRI-HF | 0.5ul | 0.5ul | 0.5ul | 0.5ul |
| XbaI | 0.5ul | 0.5ul | 0.5ul | 0.5ul |
| ddH2O | 0ul | 0ul | 0ul | 0ul |
| Total | 30ul | 30ul | 30ul | 30ul |
Incubate at 37℃ overnight. (Start from 2:30)
Go Gel Electrophoresis and Gel Extraction of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤
Time: 8:10
Handler: Lu Shixin, Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Results:
Concentration: ⑥+⑤: 25.1 ng/ul 1.58; ⑦+⑤: 23.4 ng/ul 1.54; ⑧+⑤: 45.9 ng/ul 1.01; ⑨+⑤: 26.7 ng/ul 1.50;
Ligation of ⑩,11 and ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤
Time: 11:00
Handler: Lu Shixin, Tang Shiqiang
Procedure:
- Make ligation system solution.
| ⑩+⑥+⑤ | ⑩+⑦+⑤ | ⑩+⑧+⑤ | ⑩+⑨+⑤ | |
|---|---|---|---|---|
| Vector ⑩ (50ng) | 0.8ul | 0.8ul | 0.8ul | 0.8ul |
| Insert | 3.4ul | 3.4ul | 2ul | 3.4ul |
| ddH2O | 5.8ul | 5.8ul | 7.2ul | 5.8ul |
| 2x Quick ligase buffer | 10ul | 10ul | 10ul | 10ul |
| Quick ligase | 1ul | 1ul | 1ul | 1ul |
| Total | 21ul | 21ul | 21ul | 21ul |
| 11+⑥+⑤ | 11+⑦+⑤ | 11+⑧+⑤ | 11+⑨+⑤ | |
|---|---|---|---|---|
| Vector ⑩ (50ng) | 0.8ul | 0.8ul | 0.8ul | 0.8ul |
| Insert | 3.4ul | 3.4ul | 2ul | 3.4ul |
| ddH2O | 5.8ul | 5.8ul | 7.2ul | 5.8ul |
| 2x Quick ligase buffer | 10ul | 10ul | 10ul | 10ul |
| Quick ligase | 1ul | 1ul | 1ul | 1ul |
| Total | 21ul | 21ul | 21ul | 21ul |
- Incubate at room temperature for 10 min.
- Take 5ul ligation system solution to do transformation.
8.6
Observation of single colonies of ⑩+⑥+⑤, ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑦+⑤, 11+⑧+⑤, 11+⑨+⑤
Time: 7:00
Handler: Xiao Tianyao
Results:
| Colonies number | |
|---|---|
| ⑩ backbone control | 5 |
| 11 backbone control | 20 |
| ⑩+⑥+⑤ | None |
| ⑩+⑦+⑤ | 1 |
| ⑩+⑧+⑤ | 20 |
| ⑩+⑨+⑤ | >50 |
| 11+⑥+⑤ | 4 |
| 11+⑦+⑤ | 1 |
| 11+⑧+⑤ | 6 |
| 11+⑨+⑤ | >50 |
Comments: Control groups have colonies. So the ligation results are improbable. We would redo it.
Picking single colonies of ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑦+⑤, 11+⑧+⑤, 11+⑨+⑤
Time: 10:00
Handler: Liao Weiduo
Procedure:
- Pick single colonies, adding 3 ml C+ LB.
- Incubate at 37℃ for about 12h, 220rpm.
Picking single colonies of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤
Time: 11:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics, each plate we pick 2 single colonies, C and D.
- Incubate at 37℃ for about 12h, 220rpm.
Plasmid extraction of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤ A and B
Time: 23:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ⑥+⑤C: 63.8 ng/ul 1.81; ⑥+⑤D: 59.4 ng/ul 1.79; ⑦+⑤C: 49.9 ng/ul 1.81; ⑦+⑤D: 65.8 ng/ul 1.75; ⑧+⑤C: 73.4ng/ul 1.81; ⑧+⑤D: 72.8 ng/ul 1.81; ⑨+⑤C: 65.8 ng/ul 1.74; ⑨+⑤D: 64.1 ng/ul 1.83.
8.7
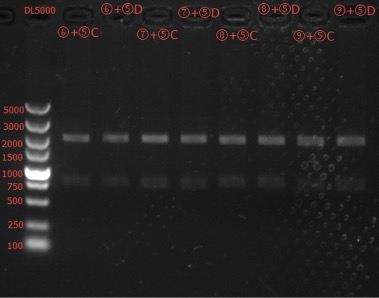
Enzyme Digestion and Go Gel Electrophoresis Test of ⑥+⑤, ⑦+⑤, ⑧+⑤, ⑨+⑤ C and D
Time: 2:00
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| ⑥+⑤C | ⑦+⑤C | ⑧+⑤C | ⑨+⑤C | ⑥+⑤D | ⑦+⑤D | ⑧+⑤D | ⑨+⑤D | |
|---|---|---|---|---|---|---|---|---|
| DNA(100ng) | 1.5ul | 1.5ul | 1.5ul | 1.5ul | 1.5ul | 1.5ul | 1.5ul | 1.5ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| XbaI | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| PstI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 16.1ul | 16.1ul | 16.1ul | 16.1ul | 16.1ul | 16.1ul | 16.1ul | 1.16ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
- Incubate at 37℃ for 30 min.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
Observation of LB medium color of ⑩+⑥+⑤, ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑦+⑤, 11+⑧+⑤, 11+⑨+⑤
Time: 7:00
Handler: Xiao Tianyao
Results:
| Color | |
|---|---|
| ⑩+⑥+⑤ | None |
| ⑩+⑦+⑤ | Purple |
| ⑩+⑧+⑤ | Yellow |
| ⑩+⑨+⑤ | Red |
| 11+⑥+⑤ | Red |
| 11+⑦+⑤ | None |
| 11+⑧+⑤ | None |
| 11+⑨+⑤ | None |
‘’’Note:’’’ There are yellow colonies on plate 11+⑧+⑤ and red colonies on 11+⑨+⑤.
Picking single colonies of ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑧+⑤, 11+⑨+⑤ (Because there are obvious correct color colonies on those plates)
Time: 9:30
Handler: Liao Weiduo
Procedure:
- Pick single colonies, adding 6 ml C+ LB.
- Incubate at 37℃ for about 12h, 220rpm.
Ligation of ⑩ and ⑥+⑤, 11 and ⑦+⑤
Time: 11:00
Handler: Tang Shiqiang
Procedure:
- Make ligation system solution.
| ⑩+⑥+⑤ | |
|---|---|
| Vector ⑩ (50ng) | 0.7ul |
| Insert | 3.2ul |
| ddH2O | 6.1ul |
| 2x Quick ligase buffer | 10ul |
| Quick ligase | 1ul |
| Total | 21ul |
| 11+⑦+⑤ | |
|---|---|
| Vector ⑩ (50ng) | 0.7ul |
| Insert | 3.2ul |
| ddH2O | 6.1ul |
| 2x Quick ligase buffer | 10ul |
| Quick ligase | 1ul |
| Total | 21ul |
- Incubate at room temperature for 10 min.
- Take 5ul ligation system solution to do transformation.
Plasmid extraction of ⑩+⑦+⑤, ⑩+⑧+⑤, ⑩+⑨+⑤, 11+⑥+⑤, 11+⑧+⑤, 11+⑨+⑤
Time: 22:00
Handler: Liao Weiduo
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ⑩+⑦+⑤: 63.6 ng/ul 1.71; ⑩+⑧+⑤: 100.7 ng/ul 1.54; ⑩+⑨+⑤: 83.0 ng/ul 1.59; 11+⑥+⑤: 114.8 ng/ul 1.65; 11+⑧+⑤: 77.5 ng/ul 1.78; 11+⑨+⑤: 106.3 ng/ul 1.51.
8.9
Picking single colonies of ⑩+⑥+⑤, 11+⑦+⑤
Time: 00:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate overnight at 37℃, 220rpm.
Plasmid extraction of ⑩+⑥+⑤, 11+⑦+⑤
Time: 16:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Results:
Concentration: ⑩+⑥+⑤: 109.0 ng/ul 1.74; 11+⑦+⑤: 115.3 ng/ul 1.85.
sfGFG
8.2
Plate streaking of a plasmid with contain sfGFP and msfGFP
Time: 17:30
Handler: Tang Shiqiang
8.3
Picking single colonies of sfGFP and msfGFP
Time: 9:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate for about 12h at 37℃, 220rpm.
Plasmid extraction of sfGFP and msfGFP
Time: 21:30
Handler: Lu Shixin
Procedure:bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: sfGFP: 70.0 ng/ul 1.77; msfGFP: 460.8 ng/ul.
8.16
PCR of sfGFP and msfGFP
Time: 16:00
Handler: Tang Shiqiang
Procedure:
| DNA | 0.1ul |
| bbp3 | 2.5ul |
| bbp4 | 2.5ul |
| Q5 | 25ul |
| ddH2O | 20ul |
| Total | 50ul |
| 32 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 5s |
| Annealing | 65℃ | 20s |
| Extension | 72℃ | 10s |
| Final elongation | 72℃ | 2min |
Go Gel Electrophoresis and Gel Extraction of sfGFP and msfGFP PCR product
Time: 19:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Result:
Concentration: sfGFP: 43.2 ng/ul 1.76; msfGFP: 62.0 ng/ul 1.79.
Enzyme Digestion for Gel Extraction of sfGFP and msfGFP
Time: 19:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| sfGFP | msfGFP | |
|---|---|---|
| DNA(>1ug) | 27.5ul | 27.5ul |
| Cutsmart buffer | 2.5ul | 2.5ul |
| EcoRI-HF | 0.5ul | 0.5ul |
| SpeI-HF | 0.5ul | 0.5ul |
| ddH2O | 19ul | 19ul |
| Total | 50ul | 50ul |
- Incubate at 37℃ for 3h.
Enzyme Digestion for Gel Extraction of ⑤
Time: 17:00
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| ⑤ | |
|---|---|
| DNA(>3ug) | 21.5ul |
| Cutsmart buffer | 2.5ul |
| EcoRI-HF | 0.5ul |
| SpeI-HF | 0.5ul |
| ddH2O | 0 |
| Total | 25ul |
- Incubate at 37℃ for 3h
Go Gel Electrophoresis and Gel Extraction of sfGFP, msfGFP, and ⑤
Time: 20:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Results:
Gel extraction concentration: ⑤: 61.4 ng/ul 1.58; sfGFP: 16.9 ng/ul 1.62; msfGFP: 16.5 ng/ul 1.59.
8.17
Ligation of sfGFP+⑤, msfGFP+⑤, sfGFP+pSB1C3, msfGFP+pSB1C3
Time: 0:00
Handler: Tang Shiqiang
Procedure:
- Make ligation system solution.
| sfGFP | msfGFP | |
|---|---|---|
| Vector ⑤ (50ng) | 1ul | 1ul |
| Insert | 4ul | 4ul |
| ddH2O | 0ul | 0ul |
| 2x Quick ligase buffer | 5ul | 5ul |
| Quick ligase | 0.5ul | 0.5ul |
| Total | 10.5ul | 10.5ul |
- Incubate at room temperature for 10 min.
- Take 10.5ul ligation system solution to do transformation.
Picking single colonies of sfGFP and msfGFP
Time: 18:30
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate overnight at 37℃, 220rpm.
8.18
Plasmid extraction of sfGFP+⑤, msfGFP+⑤, sfGFP+pSB1C3, msfGFP+pSB1C3
Time: 13:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: sfGFP+⑤: 103.6 ng/ul 1.96; msfGFP+⑤: 127.1 ng/ul 1.80; sfGFP+pSB1C3①: 156.1 ng/ul 1.91; sfGFP+pSB1C3②: 179.2 ng/ul 1.91; msfGFP+pSB1C3①: 115.5 ng/ul 1.93; msfGFP+pSB1C3②: 159.5 ng/ul 1.92.
8.18
Enzyme Digestion and Go Gel Electrophoresis Test of sfGFP+⑤, msfGFP+⑤, sfGFP+pSB1C3, msfGFP+pSB1C3
Time: 0:00
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| sfGFP+⑤ | msfGFP+⑤ | sfGFP+pSB1C3① | sfGFP+pSB1C3② | msfGFP+pSB1C3① | msfGFP+pSB1C3② | |
|---|---|---|---|---|---|---|
| DNA(200ng) | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| KpnI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 16.8ul | 16.8ul | 16.8ul | 16.8ul | 16.8ul | 16.8ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
| sfGFP+⑤ | msfGFP+⑤ | sfGFP+pSB1C3① | sfGFP+pSB1C3② | msfGFP+pSB1C3① | msfGFP+pSB1C3② | |
|---|---|---|---|---|---|---|
| DNA(200ng) | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| EcoRI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 16.8ul | 16.8ul | 16.8ul | 16.8ul | 16.8ul | 16.8ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
| sfGFP+⑤ | msfGFP+⑤ | sfGFP+pSB1C3① | sfGFP+pSB1C3② | msfGFP+pSB1C3① | msfGFP+pSB1C3② | |
|---|---|---|---|---|---|---|
| DNA(200ng) | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| Cutsmart buffer | 2ul | 2ul | 2ul | 2ul | 2ul | 2ul |
| EcoRI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| PstI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 16.6ul | 16.6ul | 16.6ul | 16.6ul | 16.6ul | 16.6ul |
| Total | 20ul | 20ul | 20ul | 20ul | 20ul | 20ul |
- Incubate overnight at 37℃.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
9.21
PCR of msfGFP Coding sequence basic part
Time: 16:00
Handler: Tang Shiqiang
Procedure:
| DNA | 1ul |
|---|---|
| SfGFP-forward | 1ul |
| bbp4 | 1ul |
| primeSTAR | 20ul |
| ddH2O | 17ul |
| Total | 40ul |
| 32 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 10s |
| Annealing | 65℃ | 5s |
| Extension | 72℃ | 5s |
| Final elongation | 72℃ | 2min |
Go Gel Electrophoresis and Gel Extraction of msfGFP CDS
Time: 20:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Result:
Concentration: msfGFP: 96.1 ng/ul 1.83.
Enzyme Digestion for Gel Extraction of msfGFP CDS
Time: 20:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| msfGFP CDS | |
|---|---|
| DNA | 43ul |
| Cutsmart buffer | 5ul |
| EcoRI-HF | 1ul |
| SpeI-HF | 1ul |
| ddH2O | 0ul |
| Total | 50ul |
- Incubate overnight at 37℃.
9.22
Transformation of msfGFP CDS
Time: 11:00
Handler: Tang Shiqiang
Procedure: IGEM Protocols
Picking single colonies of sfGFP CDS
Time: 23:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 5ml C+ LB.
- Incubate overnight at 37℃, 220rpm.
9.23
Plasmid extraction of msfGFP CDS
Time: 17:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: msfGFP CDS①: 141.0 ng/ul 1.88; msfGFP CDS②: 141.0 ng/ul 1.90.
9.24
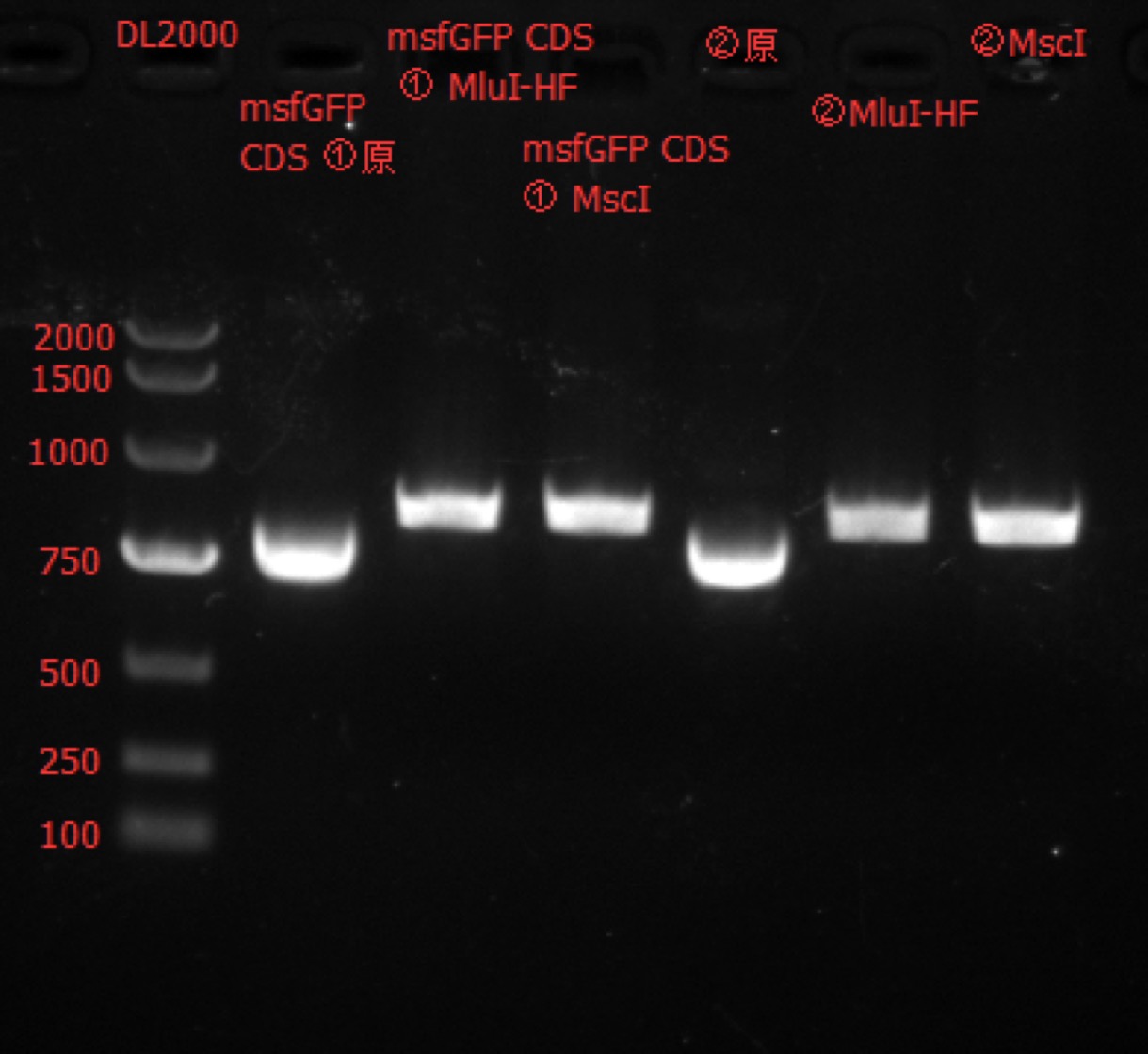
Enzyme Digestion and Go Gel Electrophoresis Test of msfGFP CDS① and msfGFP CDS②
Time: 11:40
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| msfGFP CDS① | msfGFP CDS② | |
|---|---|---|
| DNA(300ng) | 2.5ul | 2.5ul |
| Cutsmart buffer | 1ul | 1ul |
| MluI-HF/MscI | 0.5ul | 0.5ul |
| ddH2O | 6ul | 6ul |
| Total | 10ul | 10ul |
- Incubate for 3h at 37℃.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
Eukaryon Antibiotic
8.31
PCR of bla, bleo, puro, zeo
Time: 16:00
Handler: Tang Shiqiang
Procedure:
| DNA | 0.5ul |
|---|---|
| Forward | 1.25ul |
| Reverse | 1.25ul |
| PrimeSTAR | 25ul |
| ddH2O | 22ul |
| Total | 50ul |
| 33 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 10s |
| Annealing | 55℃ | 15s |
| Extension | 72℃ | 5s |
| Final elongation | 72℃ | 2min |
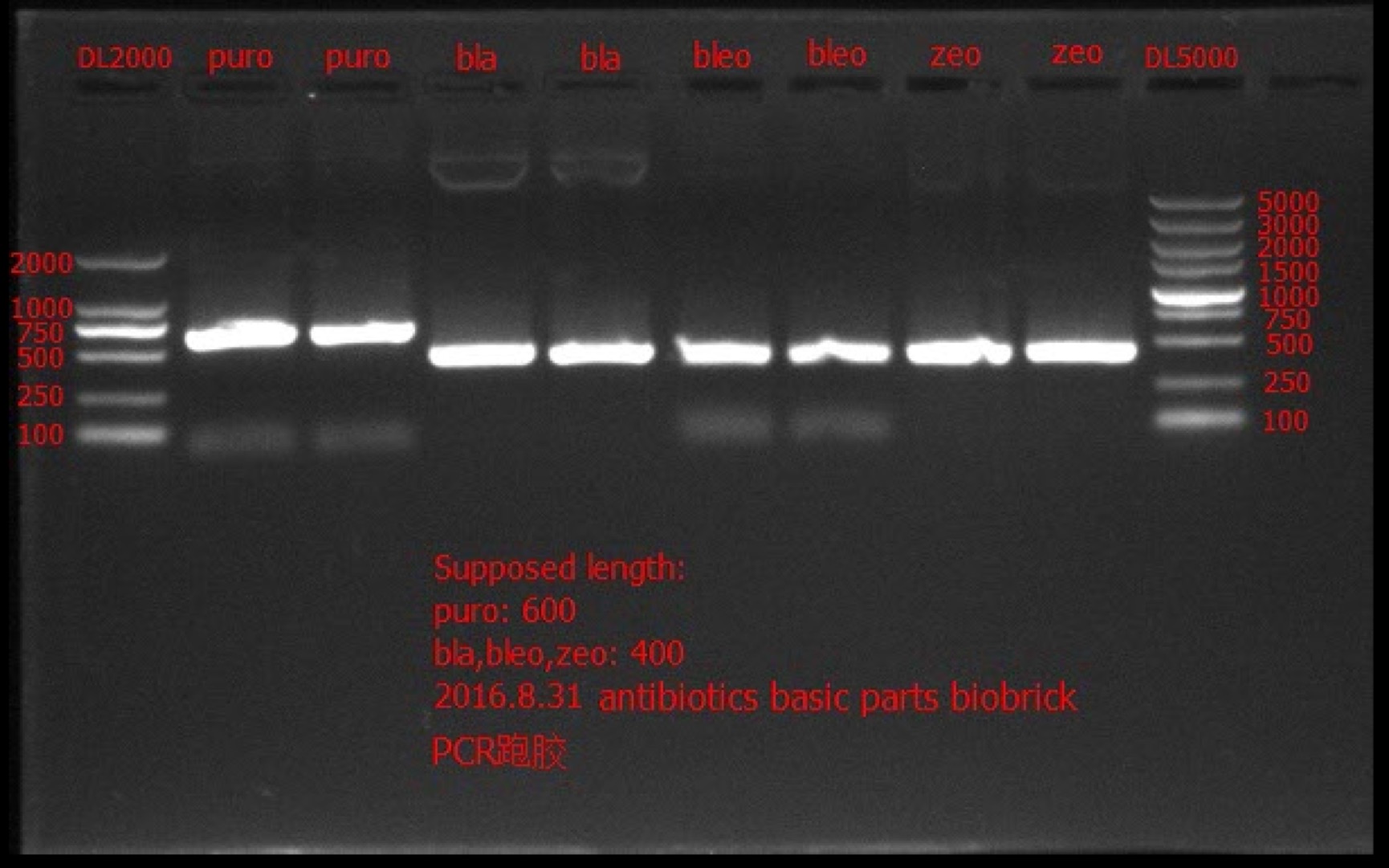
Go Gel Electrophoresis and Gel Extraction of bla, bleo, puro, zeo PCR product
Time: 18:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Result:
Concentration: bla: 66.6 ng/ul 1.91; bleo: 41.2 ng/ul 1.88; puro: 52.7 ng/ul 1.99; zeo: 46.3 ng/ul 1.97.
Enzyme Digestion for Gel Extraction of bla, bleo, puro, zeo
Time: 19:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| bla | bleo | puro | zeo | |
|---|---|---|---|---|
| DNA(>1ug) | 28ul | 28ul | 28ul | 28ul |
| Cutsmart buffer | 5ul | 5ul | 5ul | 5ul |
| EcoRI-HF | 1ul | 1ul | 1ul | 1ul |
| SpeI-HF | 1ul | 1ul | 1ul | 1ul |
| ddH2O | 15ul | 15ul | 15ul | 15ul |
| Total | 50ul | 50ul | 50ul | 50ul |
- Incubate at 37℃ for 4h.
Cycle Pure of bla, bleo, puro, zeo
Time: 23:30
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Cycle Pure Kit Centrifugation Protocol
- Transfer the PCR sample into a clean 1.5ml microcentrifuge tube.
- Add 4-5 volumes CP Buffer.
- Vortex to mix thoroughly.
- Insert a HiBind DNA Mini Column into a 2ml Collection Tube.
- Add the sample from Step 3 to the HiBind DNA Min Column.
- Centrifuge at maximum speed (>13,000 g) for 1 min at room temperature.
- Discard the filtrate and reuse collection tube.
- Add 700ul DNA Wash Buffer.
- Centrifuge at maximum speed for 1 min.
- Discard the filtrate and reuse collection tube.
- Repeat Step 8-10.
- Centrifuge the empty HiBind DNA Mini Column at maximum speed for 2 min.
- Transfer the HiBind DNA Mini Column into a clean 1.5ml microcentrifuge tube.
- Add 30-50ul ddH2O.
- Let sit at room temperature for 2 min.
- Centrifuge at maximum speed for 1 min.
Result:
Concentration: bla: 46.2 ng/ul 1.74; bleo: 20.3 ng/ul 1.32; puro: 32.4 ng/ul 1.50; zeo: 32.4 ng/ul 1.76.
9.2
Ligation of bla, bleo, puro, zeo to pSB1C3
Time: 20:30
Handler: Liao Weiduo
Procedure:
- Make ligation system solution.
| bla | bleo | puro | zeo | |
|---|---|---|---|---|
| Vector(50ng) | 2ul | 2ul | 2ul | 2ul |
| Insert | 1.5ul | 3ul | 3ul | 2ul |
| ddH2O | 6.5ul | 5ul | 5ul | 6ul |
| 2x Quick ligase buffer | 10ul | 10ul | 10ul | 10ul |
| Quick ligase | 1ul | 1ul | 1ul | 1ul |
| Total | 21ul | 21ul | 21ul | 21ul |
- Incubate at room temperature for 10 min.
- Take 10ul ligation system solution to do transformation.
9.3
Picking single colonies of bla, bleo, puro, zeo
Time: 18:00
Handler: Lu Shixin
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate overnight at 37℃, 220rpm.
9.4
Plasmid extraction of bla, bleo, puro, zeo
Time: 11:30
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: bla②: 154.7 ng/ul 1.82; bleo①: 27.8 ng/ul 2.03; bleo②: 176.8 ng/ul 1.84; puro①: 20.9 ng/ul 2.06; puro②: 249.4 ng/ul 1.74; zeo①: 121.4 ng/ul 1.94; zeo②: 154.2 ng/ul 1.85.
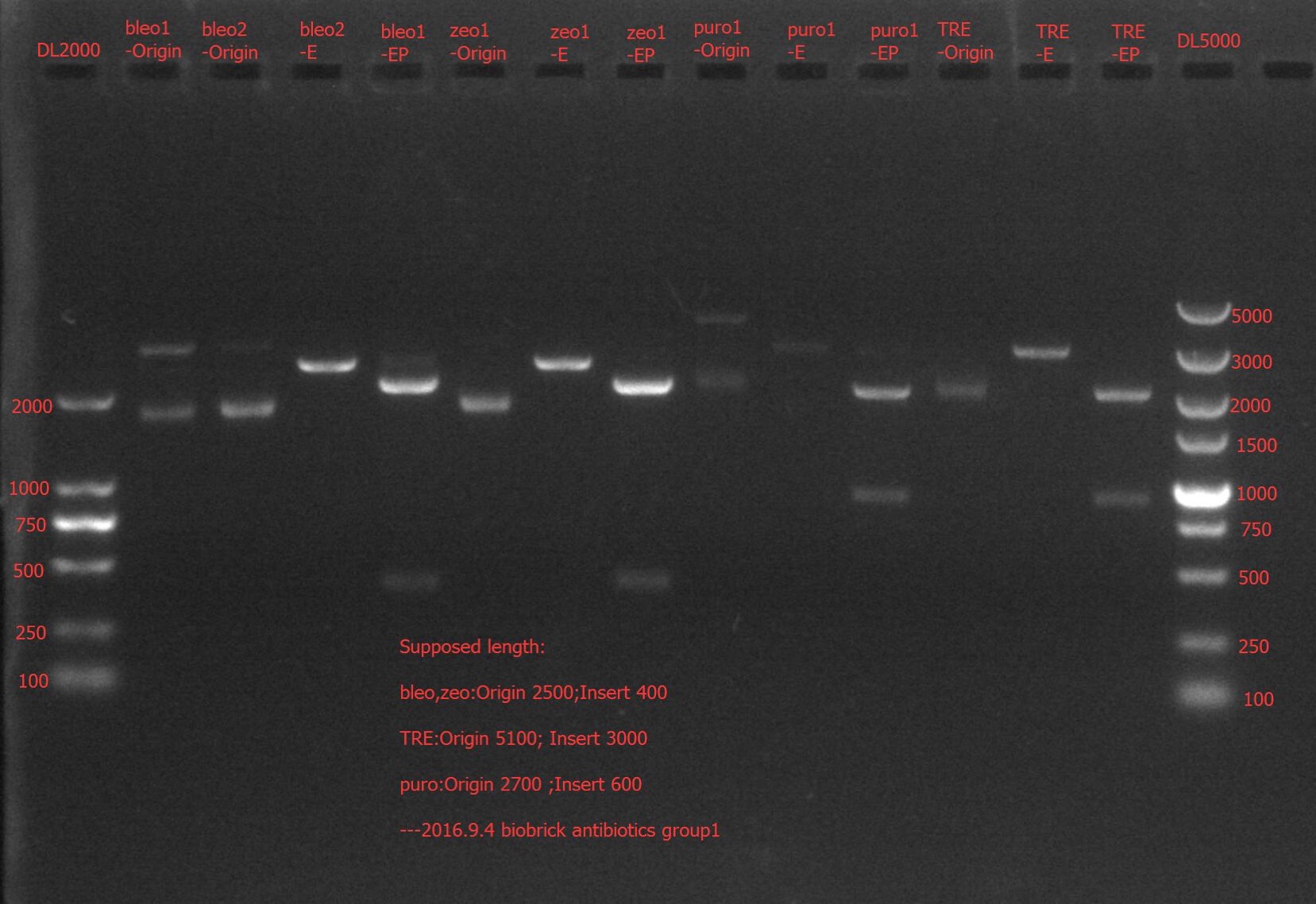
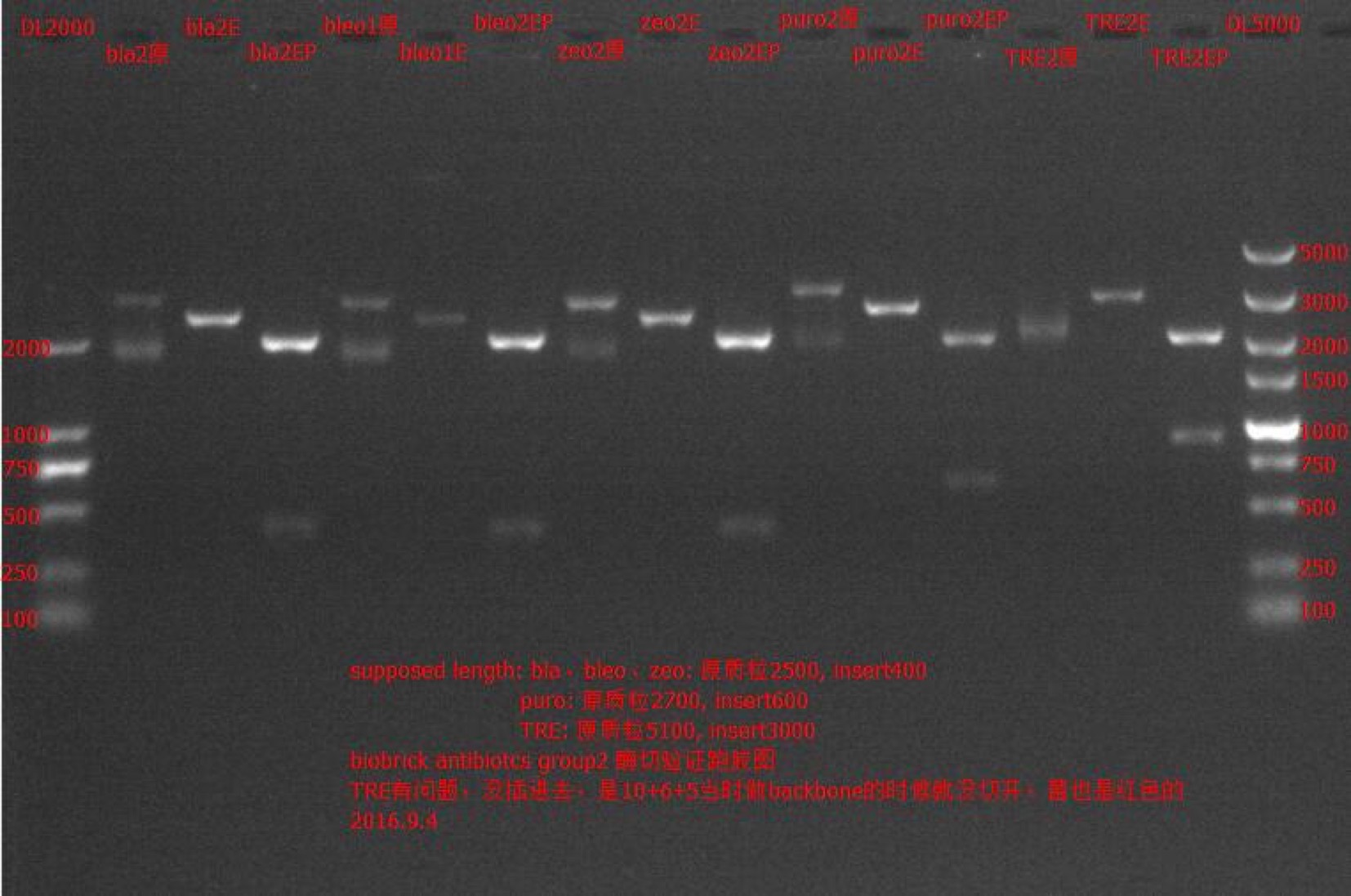
Enzyme Digestion and Go Gel Electrophoresis Test of bla, bleo, puro, zeo
Time: 17:00
Handler: Lu Shixin
Procedure:
- Make enzyme cutting system solution.
| bla② | bleo① | bleo② | puro① | puro② | zeo① | zeo② | |
|---|---|---|---|---|---|---|---|
| DNA | 2ul | 8.8ul | 2ul | 8.8ul | 1ul | 2ul | 2ul |
| Cutsmart buffer | 1ul | 1ul | 1ul | 1ul | 1ul | 1ul | 1ul |
| EcoRI-HF | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul |
| PstI-HF | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul |
| ddH2O | 6.8ul | 0ul | 6.8ul | 0ul | 7.8ul | 6.8ul | 6.8ul |
| Total | 10ul | 10ul | 10ul | 10ul | 10ul | 10ul | 10ul |
| bla② | bleo① | bleo② | puro① | puro② | zeo① | zeo② | |
|---|---|---|---|---|---|---|---|
| DNA | 1ul | 1ul | 1ul | 1ul | 1ul | 1ul | 1ul |
| Cutsmart buffer | 1ul | 1ul | 1ul | 1ul | 1ul | 1ul | 1ul |
| EcoRI-HF | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul |
| ddH2O | 7.9ul | 7.9ul | 7.9ul | 7.9ul | 7.9ul | 7.9ul | 7.9ul |
| Total | 10ul | 10ul | 10ul | 10ul | 10ul | 10ul | 10ul |
- Incubate 1h at 37℃.
- Make 1% agarose gel 50ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
9.12
Site–Directed Mutagenesis of bleo②
Time: 20:30
Handler: Tang Shiqiang
Procedure:
| DNA | 1ul |
|---|---|
| Mbleo1a | 1.25ul |
| Mbleo1b | 1.25ul |
| PrimeSTAR | 25ul |
| ddH2O | 21.5ul |
| Total | 50ul |
| 30 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 10s |
| Annealing | 55℃ | 5s |
| Extension | 72℃ | 13s |
| Final elongation | 72℃ | 2min |
Cycle Pure of mbleo
Time: 22:20
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Cycle Pure Kit Centrifugation Protocol
Result:
Concentration: mbleo: 116 ng/ul.
Enzyme Digestion for Gel Extraction of mbleo
Time: 23:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| mbleo | |
|---|---|
| DNA(>4ug) | 44.5ul |
| Cutsmart buffer | 5ul |
| DpnI | 0.5ul |
| ddH2O | 0ul |
| Total | 50ul |
- Incubate overnight at 37℃.
9.13
Transformation of mbleo
Time: 15:00
Handler: Tang Shiqiang
Procedure: IGEM Protocols
9.14
Picking single colonies of mbleo
Time: 10:30
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate about 12h at 37℃, 220rpm.
Plasmid extraction of mbleo
Time: 23:10
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: mbleo①: 104.7 ng/ul 1.89; mbleo②: 94.7 ng/ul 2.03; mbleo③: 95.4 ng/ul 1.98; mbleo④: 96.4 ng/ul 2.01.
9.15
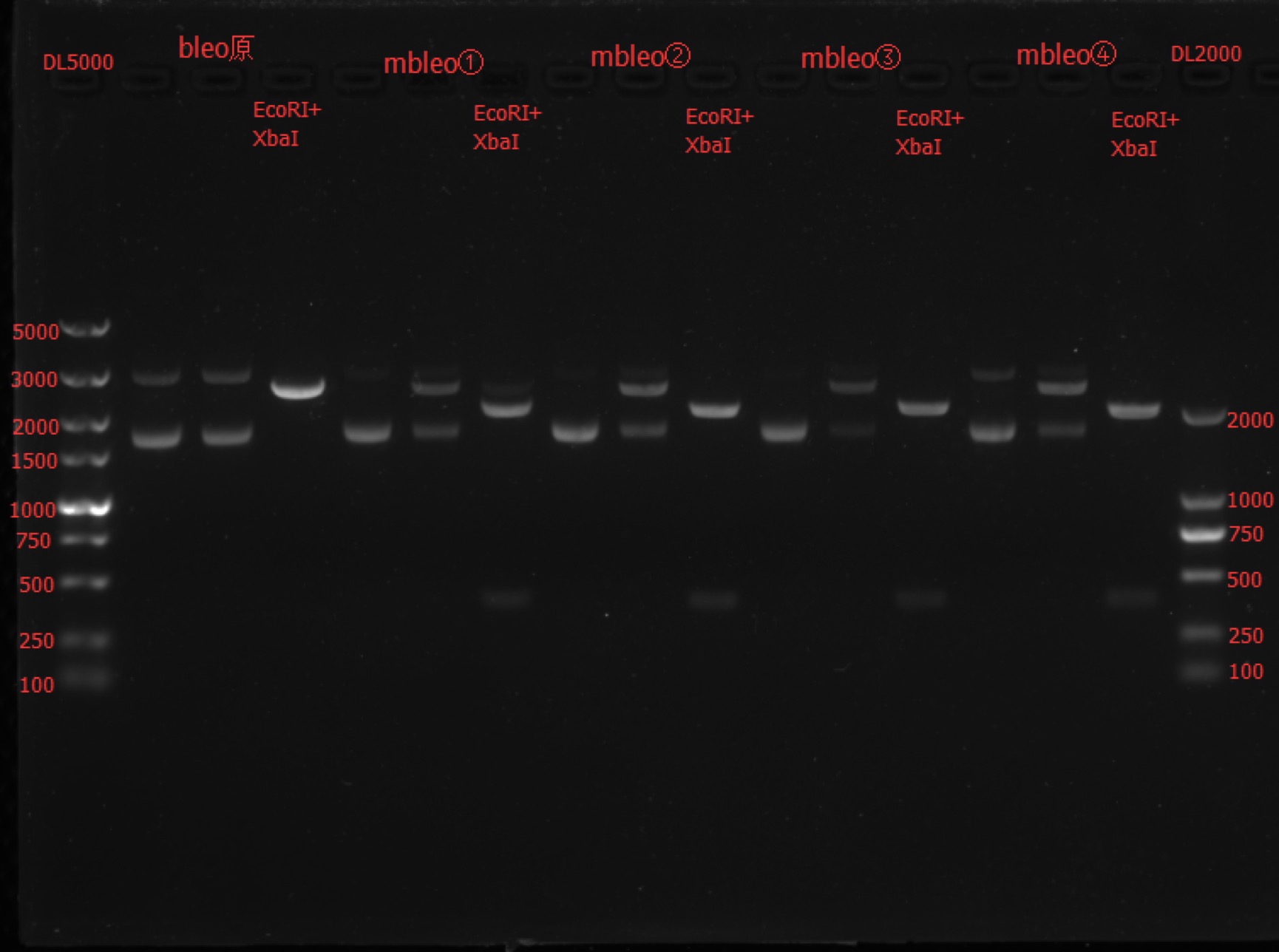
Enzyme Digestion and Go Gel Electrophoresis Test of mbleo
Time: 19:30
Handler: Lu Shixin
Procedure:
- Make enzyme cutting system solution.
| bleo | mbleo① | mbleo② | mbleo③ | mbleo④ | |
|---|---|---|---|---|---|
| DNA | 3ul | 3ul | 3ul | 3ul | 3ul |
| Cutsmart buffer | 1ul | 1ul | 1ul | 1ul | 1ul |
| XbaI | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul |
| PstI-HF | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul |
| ddH2O | 5.8ul | 5.8ul | 5.8ul | 5.8ul | 5.8ul |
| Total | 10ul | 10ul | 10ul | 10ul | 10ul |
| bleo | mbleo① | mbleo② | mbleo③ | mbleo④ | |
|---|---|---|---|---|---|
| DNA | 2ul | 2ul | 2ul | 2ul | 2ul |
| Cutsmart buffer | 1ul | 1ul | 1ul | 1ul | 1ul |
| XbaI | 0.1ul | 0.1ul | 0.1ul | 0.1ul | 0.1ul |
| ddH2O | 6.9ul | 6.9ul | 6.9ul | 6.9ul | 6.9ul |
| Total | 10ul | 10ul | 10ul | 10ul | 10ul |
- Incubate 1h at 37℃.
- Make 1% agarose gel 50ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
10.5
PCR of bla-2A, 2A-bleo, puro-2A
Time: 11:00
Handler: Tang Shiqiang
Procedure:
| DNA | 1ul |
|---|---|
| Primer1 | 1ul |
| Primer 2 | 1ul |
| PrimeSTAR | 20ul |
| ddH2O | 17ul |
| Total | 40ul |
| 30 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 10s |
| Annealing | 55℃ | 10s |
| Extension | 72℃ | 25s |
| Final elongation | 72℃ | 2min |
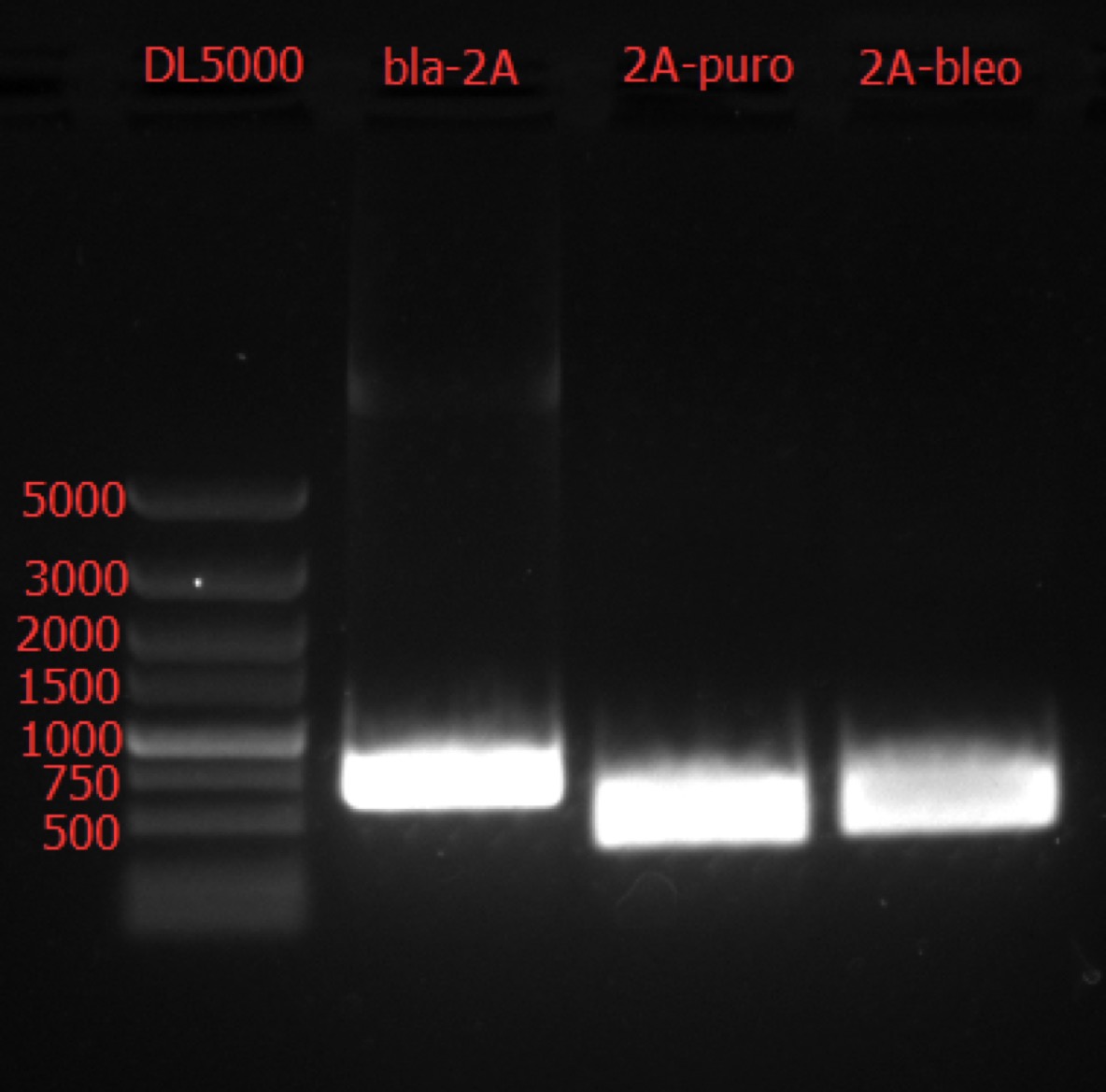
Go Gel Electrophoresis and Gel Extraction of bla-2A, 2A-bleo, puro-2A PCR Product
Time: 19:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Concentration: bla-2A: 31.1 ng/ul 3.19; 2A-bleo: 47.4 ng/ul 2.28; puro-2A: 73.0 ng/ul 2.32.
Enzyme Digestion for Gel Extraction of bla-2A, 2A-bleo, puro-2A
Time: 19:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| bla-2A | 2A-bleo | puro-2A | |
|---|---|---|---|
| DNA(>1ug) | 44ul | 44ul | 44ul |
| Cutsmart buffer | 5ul | 5ul | 5ul |
| EcoRI-HF | 0.5ul | 0.5ul | 0.5ul |
| SpeI-HF | 0.5ul | 0.5ul | 0.5ul |
| ddH2O | 0ul | 0ul | 0ul |
| Total | 50ul | 50ul | 50ul |
- Incubate for 5h at 37℃.
10.6
Cycle Pure of bla-2A, 2A-bleo, puro-2A
Time: 1:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Cycle Pure Kit Centrifugation Protocol
Results:
Concentration: bla-2A: 72.4 ng/ul 1.89; 2A-bleo: 74.4 ng/ul 1.88; puro-2A: 175.3 ng/ul 1.91.
Ligation of bla-2A, 2A-bleo, puro-2A to pSB1C3
Time: 1:30
Handler: Tang Shiqiang
Procedure:
- Make ligation system solution.
| bla-2A | 2A-bleo | puro-2A | |
|---|---|---|---|
| Vector(50ng) | 1ul | 1ul | 1ul |
| Insert | 0.7ul | 0.7ul | 0.3ul |
| ddH2O | 3.3ul | 3.3ul | 3.7ul |
| 2x Quick ligase buffer | 5ul | 5ul | 5ul |
| Quick ligase | 0.5ul | 0.5ul | 0.5ul |
| Total | 10.5ul | 10.5ul | 10.5ul |
- Incubate at room temperature for 10 min.
- Take 10ul ligation system solution to do transformation.
Picking single colonies of bla-2A, 2A-bleo, puro-2A
Time: 19:30
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate overnight at 37℃, 220rpm.
10.7
Plasmid extraction of bla-2A, 2A-bleo, puro-2A
Time: 11:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: bla-2A①: 266.9 ng/ul 1.94; bla-2A②: 249.3 ng/ul 1.95; 2A-bleo①: 261.2 ng/ul 1.95; 2A-bleo②: 224.5 ng/ul 1.96; puro-2A①: 223.6 ng/ul 1.99; puro-2A②: 170.2 ng/ul 1.98.
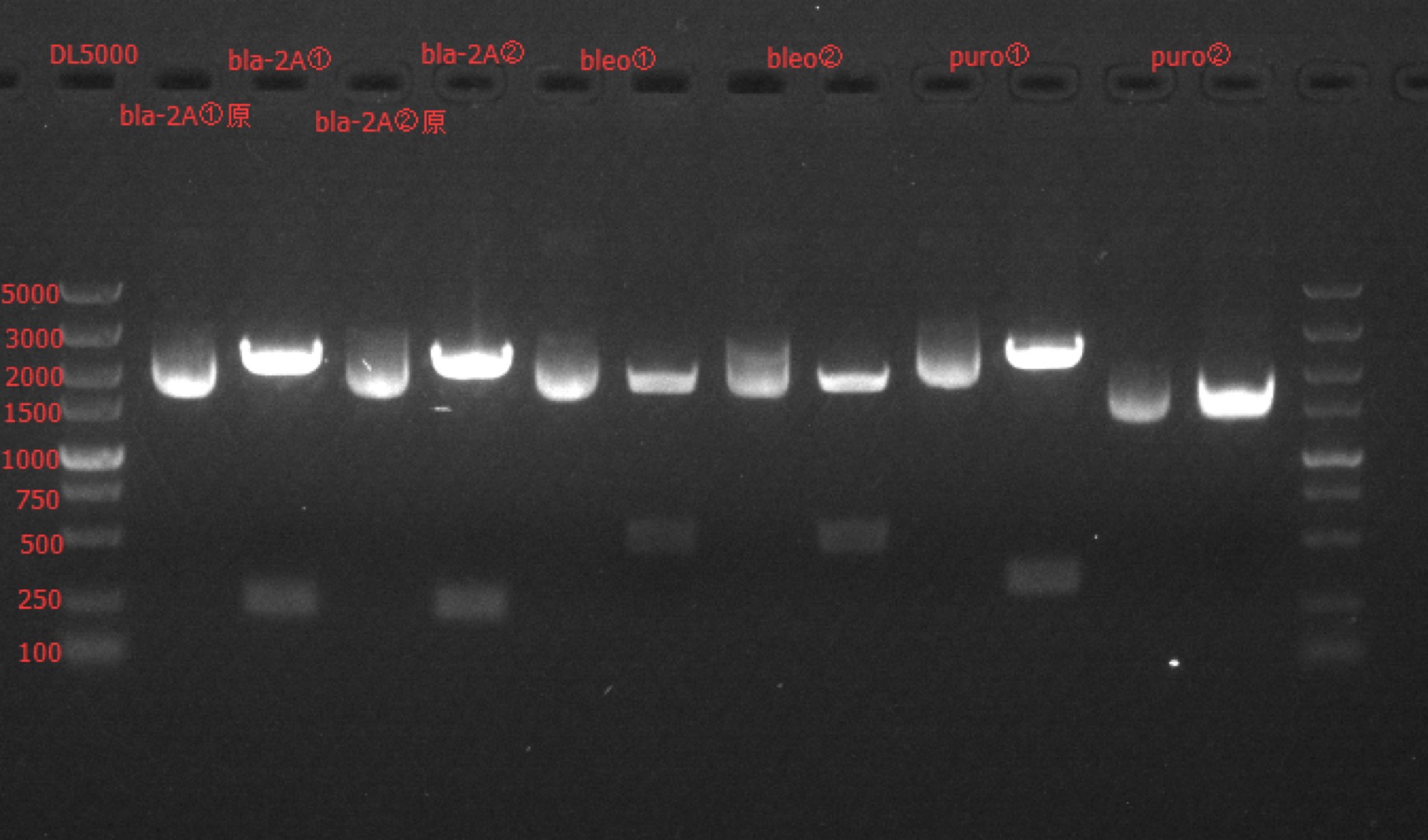
Enzyme Digestion and Go Gel Electrophoresis Test of bla-2A, 2A-bleo, puro-2A
Time: 13:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| bla-2A① | bla-2A② | 2A-bleo① | 2A-bleo② | puro-2A① | puro-2A② | |
|---|---|---|---|---|---|---|
| DNA | 3.6ul | 3.9ul | 1.3ul | 1.6ul | 3.5ul | 4.5ul |
| Cutsmart buffer | 1ul | 1ul | 1ul | 1ul | 1ul | 1ul |
| EcoRI-HF | 0.2ul | 0.2ul | \ | \ | 0.2ul | 0.2ul |
| PvuI-HF | 0.2ul | 0.2ul | \ | \ | \ | \ |
| StuI | \ | \ | \ | \ | 0.2ul | 0.2ul |
| ApaLI | \ | \ | 0.3ul | 0.3ul | \ | \ |
| ddH2O | 5ul | 4.7ul | 7.4ul | 7.1ul | 5.1ul | 4.1ul |
| Total | 10ul | 10ul | 10ul | 10ul | 10ul | 10ul |
- Incubate 1h at 37℃.
- Make 1% agarose gel 50ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
GECO
9.23
PCR of GECO
Time: 15:00
Handler: Tang Shiqiang
Procedure:
| DNA | 1ul |
|---|---|
| Forward | 1ul |
| Reverse | 1ul |
| PrimeSTAR | 20ul |
| ddH2O | 17ul |
| Total | 40ul |
| 30 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 5s |
| Annealing | 55℃ | 5s |
| Extension | 72℃ | 30s |
| Final elongation | 72℃ | 2min |
Go Gel Electrophoresis and Gel Extraction of bla, bleo, puro, zeo PCR product
Time: 19:30
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Result:
Concentration: 84.1 ng/ul 1.77.
Enzyme Digestion for Gel Extraction of GECO
Time: 20:00
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| GECO | |
|---|---|
| DNA(>3ug) | 44ul |
| Cutsmart buffer | 5ul |
| EcoRI-HF | 0.5ul |
| SpeI-HF | 0.5ul |
| ddH2O | 0ul |
| Total | 50ul |
- Incubate for 5h at 37℃.
9.24
Cycle Pure of GECO
Time: 1:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Cycle Pure Kit Centrifugation Protocol
Results:
Concentration: GECO: 133.4 ng/ul 1.85.
Ligation of GECO to pSB1C3
Time: 1:30
Handler: Tang Shiqiang
Procedure:
- Make ligation system solution.
| GECO | |
|---|---|
| Vector(50ng) | 0.4ul |
| Insert | 0.4ul |
| ddH2O | 4.2ul |
| 2x Quick ligase buffer | 5ul |
| Quick ligase | 0.5ul |
| Total | 10.5ul |
- Incubate at room temperature for 10 min.
- Take 10ul ligation system solution to do transformation.
Picking single colonies of GECO
Time: 19:30
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate overnight at 37℃, 220rpm.
9.25
Plasmid extraction of GECO
Time: 10:20
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: GECO①: 352.8 ng/ul 1.81; GECO②: 360.7 ng/ul 1.73.
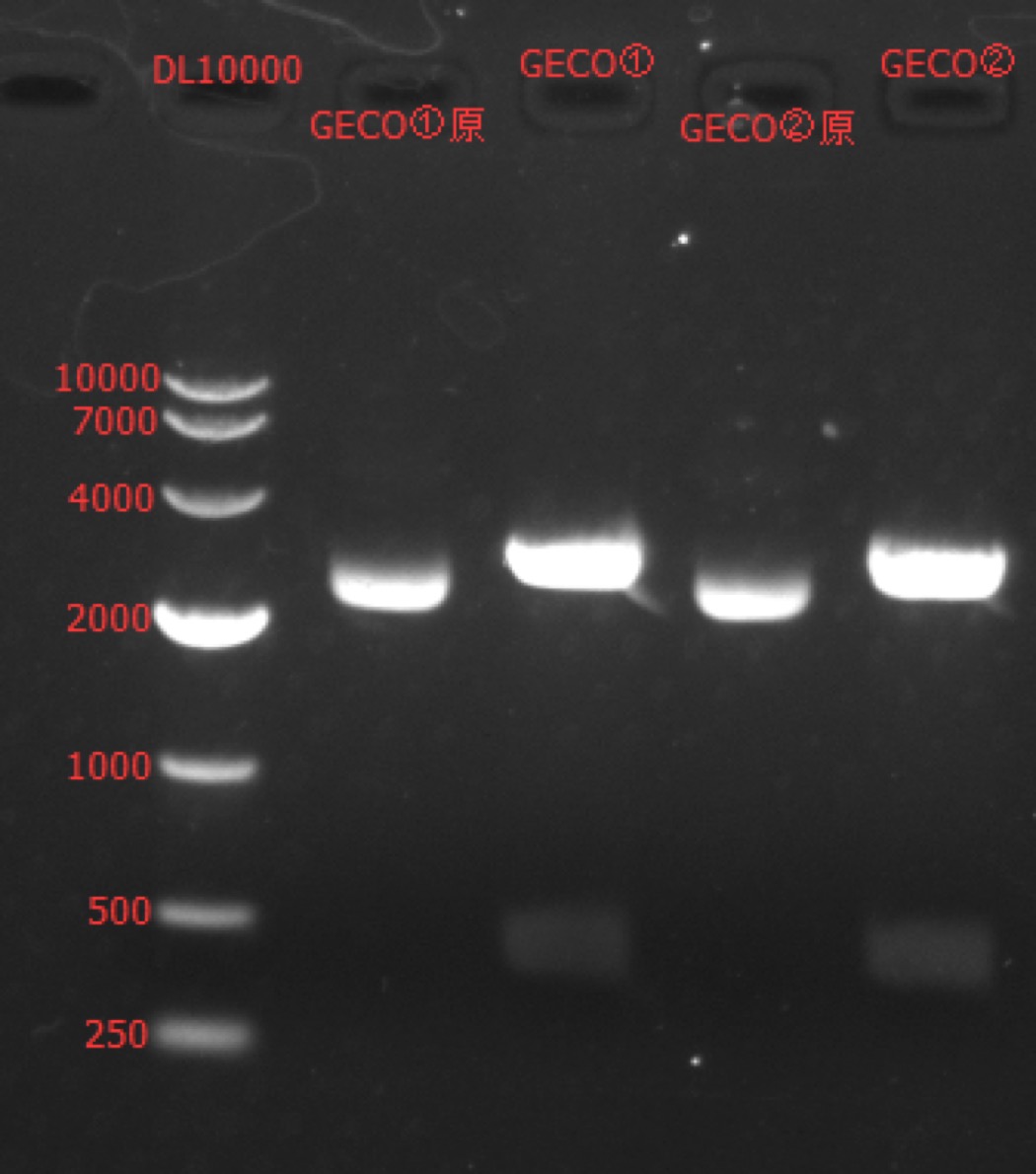
Enzyme Digestion and Go Gel Electrophoresis Test of GECO
Time: 14:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| GECO① | GECO② | |
|---|---|---|
| DNA | 2ul | 2ul |
| Cutsmart buffer | 1ul | 1ul |
| SbfI-HF | 0.5ul | 0.5ul |
| BspDI | 0.5ul | 0.5ul |
| ddH2O | 6ul | 6ul |
| Total | 10ul | 10ul |
- Incubate 1h at 37℃.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
Site–Directed Mutagenesis of GECO①
Time: 16:20
Handler: Tang Shiqiang
Procedure:
| DNA | 1ul |
| Mpst1a | 1ul |
| Mpst1b | 1ul |
| PrimeSTAR | 20ul |
| ddH2O | 17ul |
| Total | 40ul |
| 30 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 10s |
| Annealing | 55℃ | 5s |
| Extension | 72℃ | 90s |
| Final elongation | 72℃ | 2min |
Cycle Pure of M1GECO
Time: 20:00
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Cycle Pure Kit Centrifugation Protocol
Result:
Concentration: M1GECO: 118.8 ng/ul 1.83.
Enzyme Digestion for Gel Extraction of M1GECO
Time: 20:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| M1GECO | GECO control | |
|---|---|---|
| DNA(>4ug) | 44.5ul | 5ul |
| Cutsmart buffer | 5ul | 5ul |
| DpnI | 0.5ul | 0.5ul |
| ddH2O | 0ul | 43.5ul |
| Total | 50ul | 50ul |
- Incubate for 2h at 37℃.
9.26
Transformation of M1GECO
Time: 0:40
Handler: Tang Shiqiang
Procedure: IGEM Protocols
Picking single colonies of M1GECO
Time: 19:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate overnight at 37℃, 220rpm.
9.27
Plasmid extraction of M1GECO
Time: 12:00
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: M1GECO①: 262.7 ng/ul 1.71; M1GECO②: 277.1 ng/ul 1.60.
Site–Directed Mutagenesis of M1GECO
Time: 13:00
Handler: Tang Shiqiang
Procedure:
| DNA | 1ul |
|---|---|
| Mpst2a | 1ul |
| Mpst2b | 1ul |
| PrimeSTAR | 20ul |
| ddH2O | 17ul |
| Total | 40ul |
| 30 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 10s |
| Annealing | 55℃ | 5s |
| Extension | 72℃ | 90s |
| Final elongation | 72℃ | 2min |
Cycle Pure of M2GECO
Time: 17:00
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Cycle Pure Kit Centrifugation Protocol
Result:
Concentration: M2GECO: 100.0 ng/ul 1.83.
Enzyme Digestion for Gel Extraction of M2GECO
Time: 17:30
Handler: Lu Shixin
Procedure:
- Make enzyme cutting system solution.
| M2GECO | M1GECO control | |
|---|---|---|
| DNA(>4ug) | 44.5ul | 5ul |
| Cutsmart buffer | 5ul | 5ul |
| DpnI | 0.5ul | 0.5ul |
| ddH2O | 0ul | 43.5ul |
| Total | 50ul | 50ul |
- Incubate for 3h at 37℃.
Transformation of M2GECO
Time: 20:30
Handler: Lu Shixin
Procedure: IGEM Protocols
9.28
Picking single colonies of M2GECO
Time: 10:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate for 13h at 37℃, 220rpm.
Plasmid extraction of M2GECO
Time: 23:00
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: M2GECO①: 175.1 ng/ul 1.70; M2GECO②: 117.5 ng/ul 1.86; M2GECO③: 102 ng/ul 1.90.
9.29
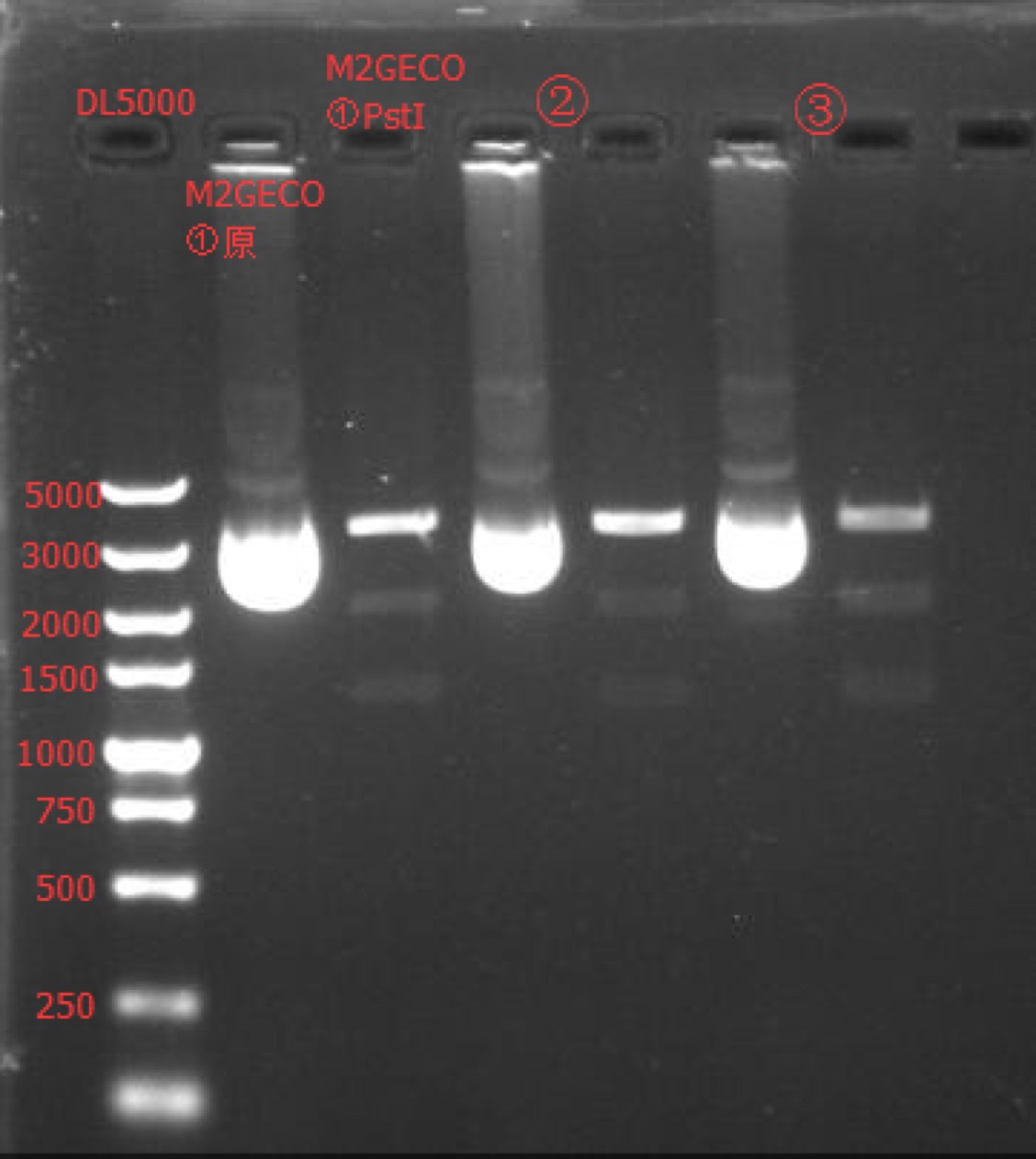
=Enzyme Digestion and Go Gel Electrophoresis Test of M2GECO
Time: 0:00
Handler: Lu Shixin
Procedure:
- Make enzyme cutting system solution.
| M2GECO① | M2GECO② | M2GECO③ | |
|---|---|---|---|
| DNA | 1ul | 1ul | 1ul |
| Cutsmart buffer | 1ul | 1ul | 1ul |
| PstI-HF | 0.1ul | 0.1ul | 0.1ul |
| ddH2O | 7.9ul | 7.9ul | 7.9ul |
| Total | 10ul | 10ul | 10ul |
- Incubate overnight at 37℃.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results:
TetOn
9.26
PCR of GECO
Time: 20:00
Handler: Tang Shiqiang
Procedure:
| DNA | 1ul |
|---|---|
| Forward | 1ul |
| Reverse | 1ul |
| PrimeSTAR | 20ul |
| ddH2O | 17ul |
| Total | 40ul |
| 30 cycles | Temperature | Time |
|---|---|---|
| Initialization | 98℃ | 30s |
| Denaturation | 98℃ | 10s |
| Annealing | 55℃ | 5s |
| Extension | 72℃ | 40s |
| Final elongation | 72℃ | 2min |
Go Gel Electrophoresis and Gel Extraction of TetOn PCR product
Time: 21:30
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Gel Extraction Kit – Spin Protocol
Result:
Concentration: TetOn: 86.7 ng/ul 1.66.
Enzyme Digestion for Gel Extraction of TetOn
Time: 22:30
Handler: Lu Shixin
Procedure:
- Make enzyme cutting system solution.
| TetOn | |
|---|---|
| DNA(>3ug) | 44ul |
| Cutsmart buffer | 5ul |
| EcoRI-HF | 0.5ul |
| SpeI-HF | 0.5ul |
| ddH2O | 0ul |
| Total | 50ul |
- Incubate overnight at 37℃.
9.27
Cycle Pure of TetOn
Time: 12:00
Handler: Lu Shixin
Procedure: bio-tek OMEGA E.Z.N.A. Cycle Pure Kit Centrifugation Protocol
Results:
Concentration: TetOn: 45.4 ng/ul 1.80.
Ligation of TetOn to pSB1C3
Time: 15:00
Handler: Tang Shiqiang
Procedure:
- Make ligation system solution.
| TetOn | |
|---|---|
| Vector(50ng) | 0.4ul |
| Insert | 1.6ul |
| ddH2O | 3ul |
| 2x Quick ligase buffer | 5ul |
| Quick ligase | 0.5ul |
| Total | 10.5ul |
- Incubate at room temperature for 10 min.
- Take 10ul ligation system solution to do transformation.
- Put the plate at 4℃ after 16h.
9.29
Picking single colonies of TetOn
Time: 20:00
Handler: Tang Shiqiang
Procedure:
- Pick single colonies, adding 6ml LB with corresponding antibiotics.
- Incubate overnight at 37℃, 220rpm.
9.30
Plasmid extraction of TetOn
Time: 10:30
Handler: Tang Shiqiang
Procedure: bio-tek OMEGA E.Z.N.A. Plasmid DNA Mini Kit I Spin Protocol
Result:
Concentration: TetOn①: 246.6 ng/ul 1.79; TetOn②: 314.1 ng/ul 1.86; TetOn③: 106.3 ng/ul 1.86; TetOn④: 298.8 ng/ul 1.86.
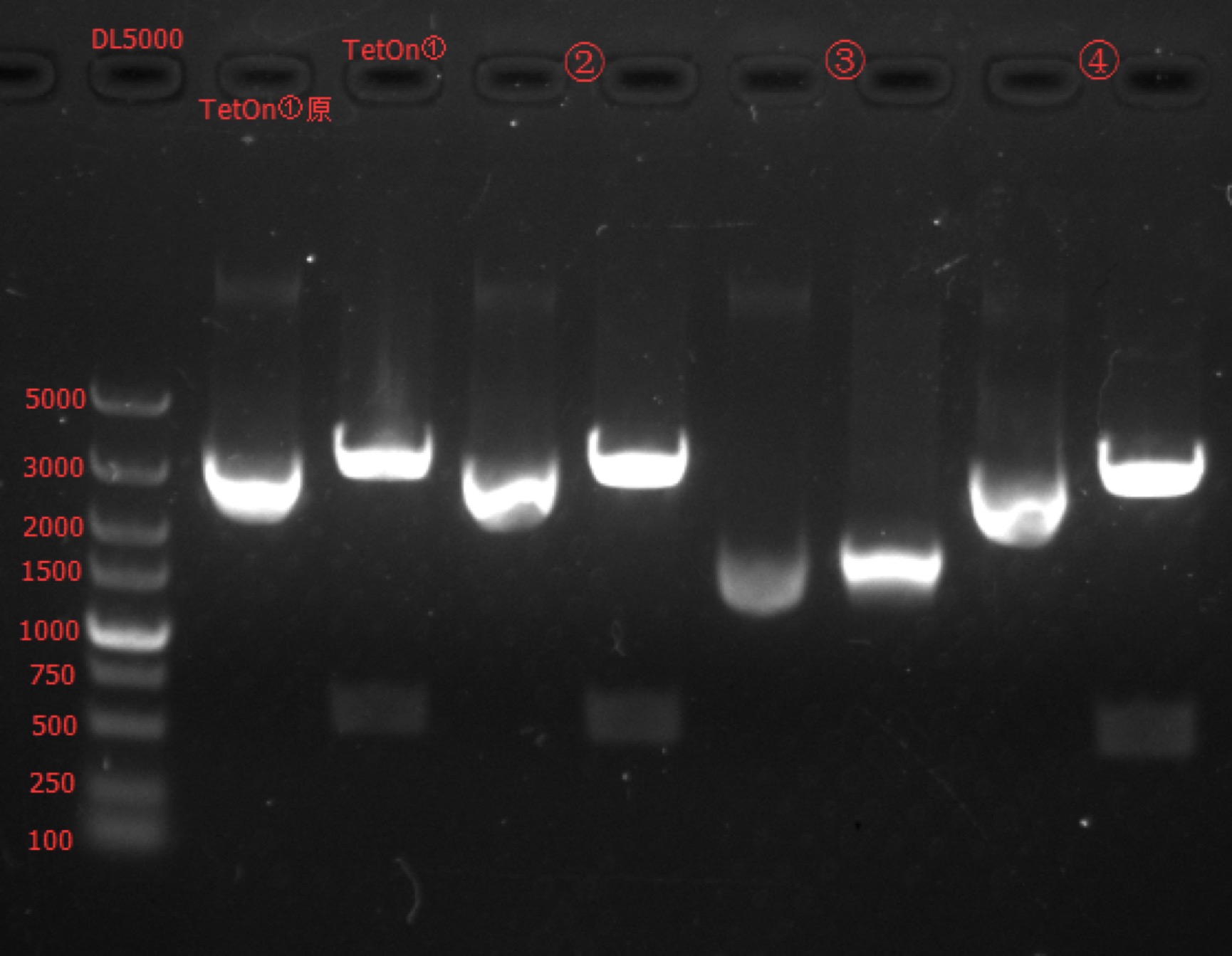
Enzyme Digestion and Go Gel Electrophoresis Test of TetOn
Time: 14:30
Handler: Tang Shiqiang
Procedure:
- Make enzyme cutting system solution.
| TetOn① | TetOn② | TetOn③ | TetOn④ | |
|---|---|---|---|---|
| DNA | 2.5ul | 2.5ul | 6ul | 2.5ul |
| Cutsmart buffer | 1ul | 1ul | 1ul | 1ul |
| EcoRI-HF | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| SacII | 0.2ul | 0.2ul | 0.2ul | 0.2ul |
| ddH2O | 6.1ul | 6.1ul | 2.6ul | 6.1ul |
| Total | 10ul | 10ul | 10ul | 10ul |
- Incubate 1h at 37℃.
- Make 1% agarose gel 30ml.
- Add 6x loading dye to the enzyme cutting system solution.
- Go gel electrophoresis.
Results: