| Line 7: | Line 7: | ||
= Overview = | = Overview = | ||
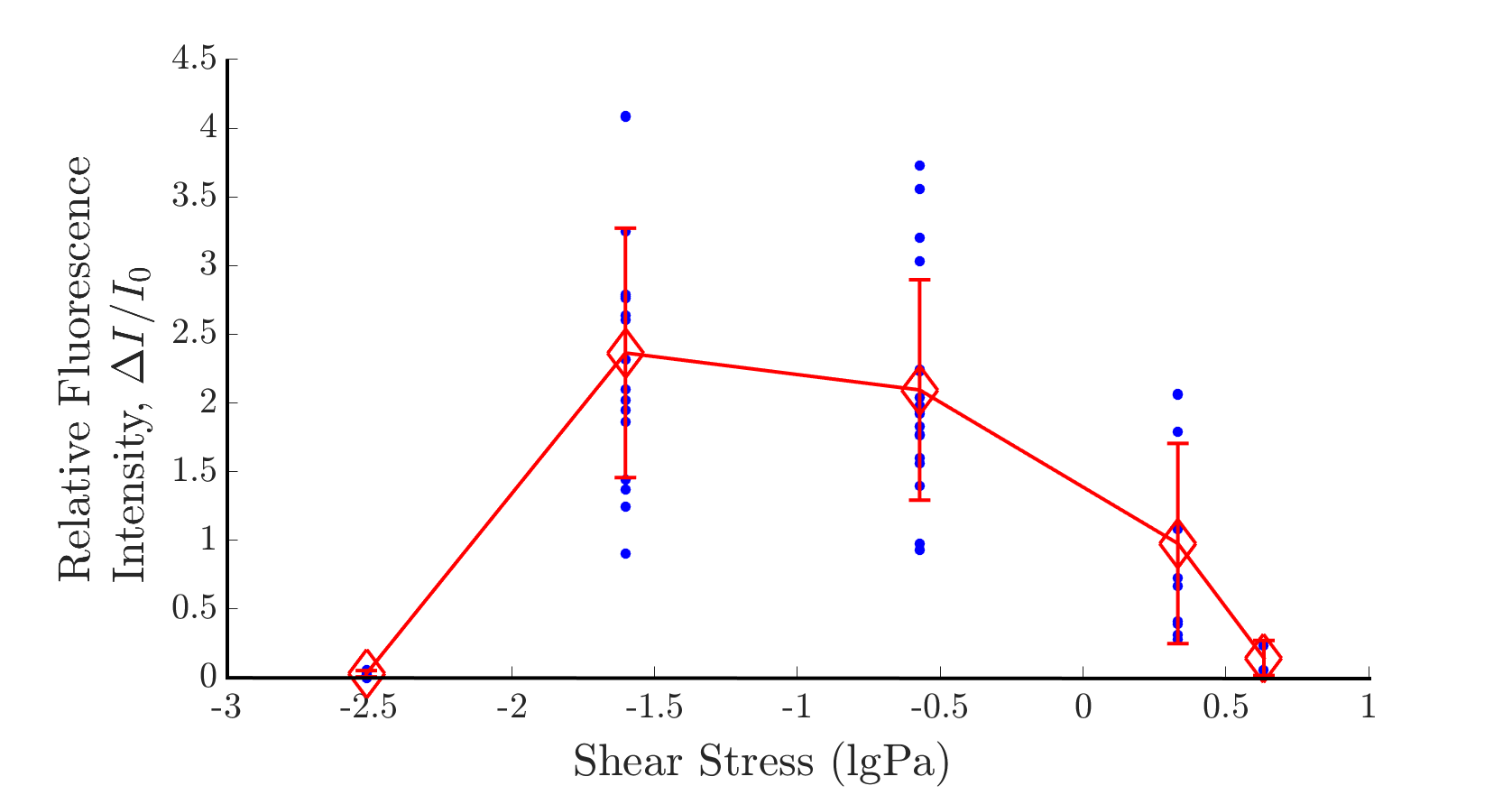
| − | Precisely controling the mechanical stress applied on cells is an important key to open the door to explore the mechanism of cells' sensation to sound vibration. However, the current research methods used for mechanically activate cells are not pay much concern on the magnitude of mechanical stress. Here we designed a microfluidic chip to quantitatively tune the shear force exerted on cell membrane, and both channel are responsive to such mechical stress. Surprisingly, we discovered that Piezo1-expression CHO cell response to shear force in an biphasic manner and a maximum responsive of ~0. | + | Precisely controling the mechanical stress applied on cells is an important key to open the door to explore the mechanism of cells' sensation to sound vibration. However, the current research methods used for mechanically activate cells are not pay much concern on the magnitude of mechanical stress. Here we designed a microfluidic chip to quantitatively tune the shear force exerted on cell membrane, and both channel are responsive to such mechical stress. Surprisingly, we discovered that Piezo1-expression CHO cell response to shear force in an biphasic manner and a maximum responsive of ~0.1 pa, which haven't found similar report in the past. |
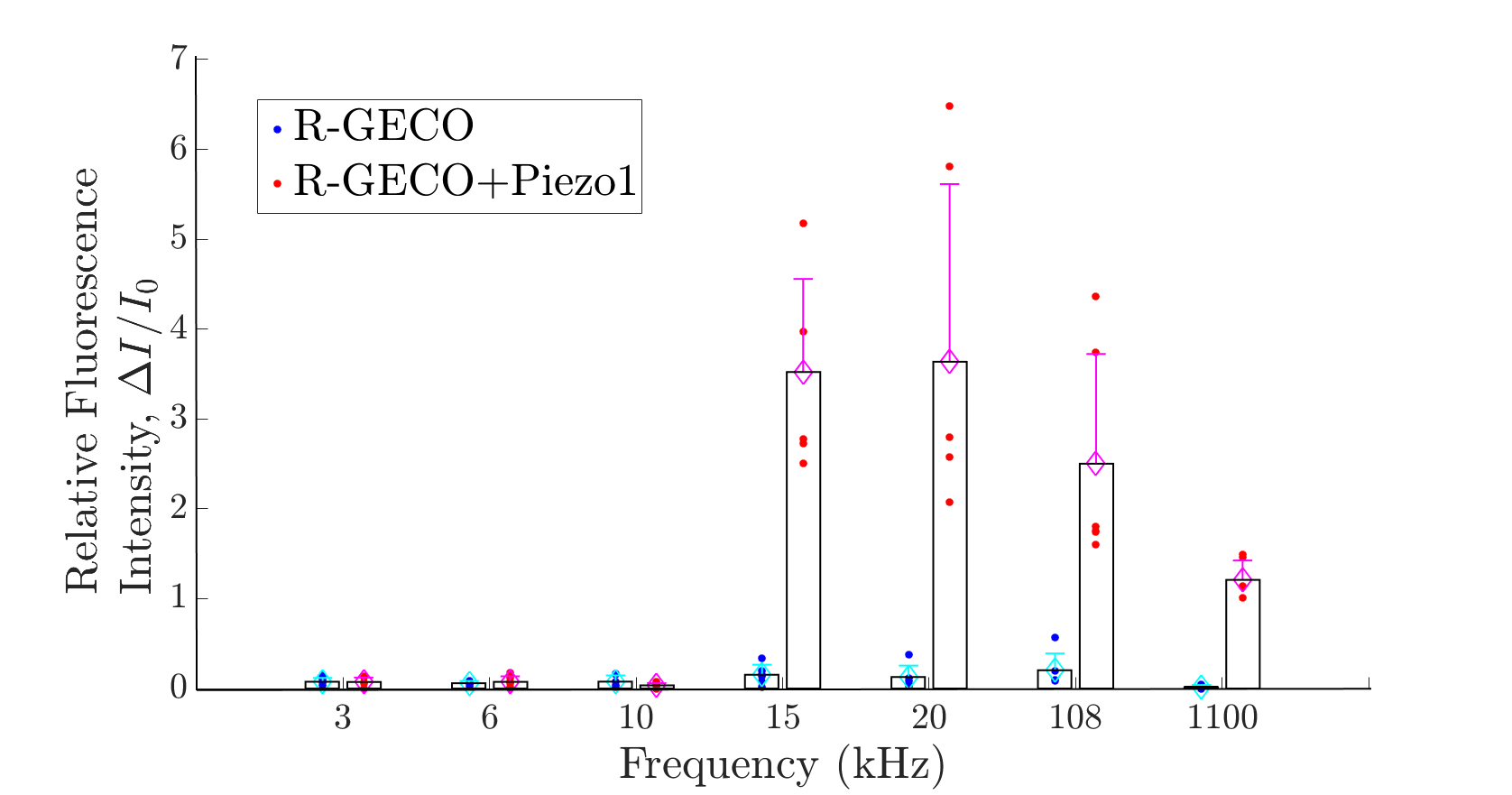
We also examined the intracellular calcium response to various combination of frequency and intensity of sound, including both the audible and ultrsound range. We discovered that Piezo1-expression CHO cell can sense the sound that above 15kHz, which indicate the previous identified touching sensing Piezo1 channel could detect sound wave of particular frequency range. | We also examined the intracellular calcium response to various combination of frequency and intensity of sound, including both the audible and ultrsound range. We discovered that Piezo1-expression CHO cell can sense the sound that above 15kHz, which indicate the previous identified touching sensing Piezo1 channel could detect sound wave of particular frequency range. | ||
= 1. Biphasic Response of Piezo1 Channel = | = 1. Biphasic Response of Piezo1 Channel = | ||
{{SUSTech_Image | filename=T--SUSTech_Shenzhen--demon-biphasic.png|caption=<B>Fig.1 Optimal range of shear response</B>| width=1000px}} | {{SUSTech_Image | filename=T--SUSTech_Shenzhen--demon-biphasic.png|caption=<B>Fig.1 Optimal range of shear response</B>| width=1000px}} | ||
| − | We found that Piezo1 channel exhibits two properties: high sensitivity at 0. | + | We found that Piezo1 channel exhibits two properties: high sensitivity at 0.1Pa, and biphasic response. To the best of our knowledge, |
both are novel discoveries. | both are novel discoveries. | ||
| Line 18: | Line 18: | ||
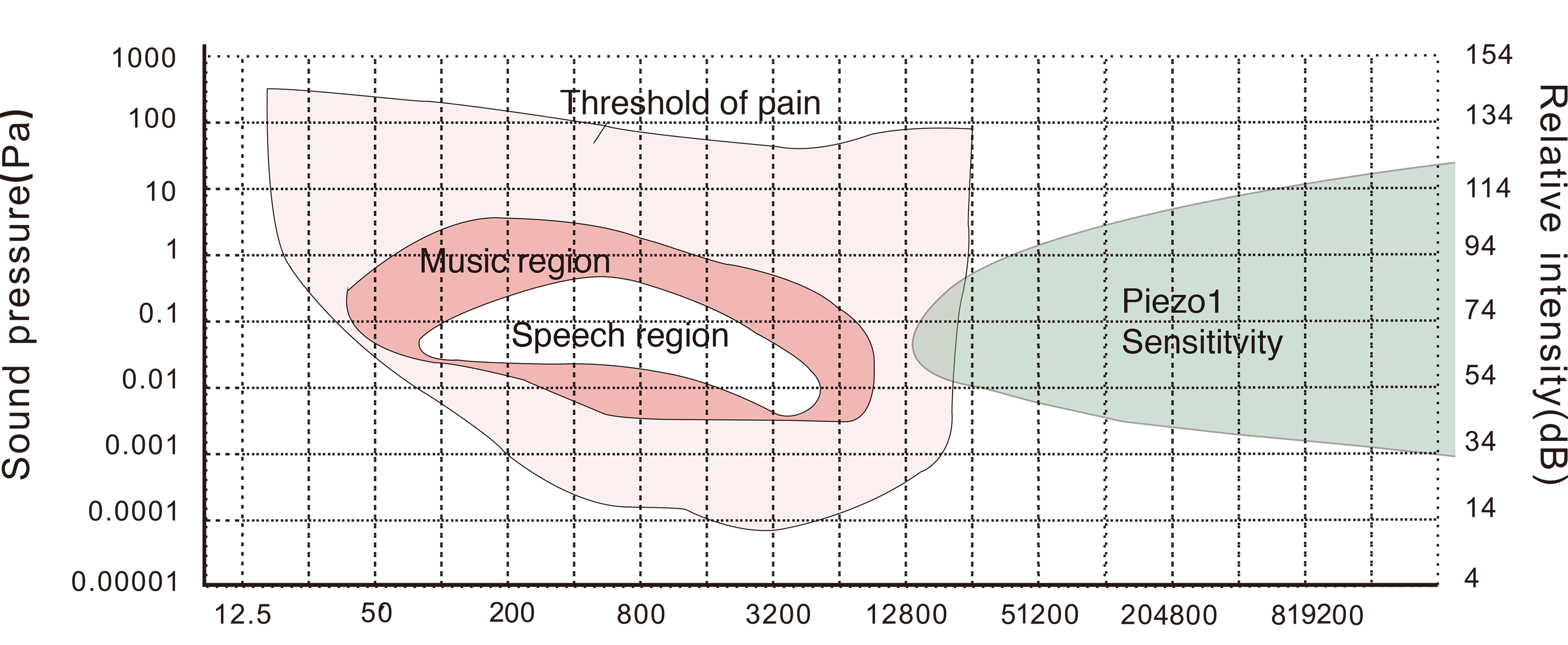
{{SUSTech_Image | filename=T--SUSTech_Shenzhen--demon-audibility.png | width=1000px|caption=<B> Fig.2B the sensible range of Piezo1 channel in the audible range</B>}} | {{SUSTech_Image | filename=T--SUSTech_Shenzhen--demon-audibility.png | width=1000px|caption=<B> Fig.2B the sensible range of Piezo1 channel in the audible range</B>}} | ||
| − | We found that Piezo1 channel can sense acoustic wave from ultrasound to the margin of human audible range. Best response is at 20kHz when using piezo buzzer as sound generator. | + | We found that Piezo1 channel can sense acoustic wave from ultrasound to the margin of human audible range. Best response is at 20kHz when using piezo buzzer as sound generator. In microfluidics, the shear stress, about 0.1 pa, apply on cell is continuously dragging Piezo1 channel to open it, however, the mechanism to stop it in higher shear stress is remains unknown. While in acoustic stimulation, the pressure generated by our designed sound wave oscillation is bigger than 100kPa, which is conflict with the result of microfluidics. After consultation with Prof. Bin Tang, who is an expert in biomechanics field. He referred that high frequency oscillation leads visco-elastic transition, in other words, makes cytoskeleton becomes harder, and stiffer cytoskeleton needs lager vibrating force to deform the cytoskeleton. |
| + | = 3. Future Plan = | ||
| + | |||
| + | The feedback regulatory motif is most common in the gene regulatory network of cells, including the hair cells. They can change mechanical thresholds by increase or decrease the expression of a mechanosensitive channel (MS) channel. Also, we have proof that downstream GFP of the NFAT (Nuclear factor of activated T-cells) promoter can be successfully induced by long term calcium signal. Thus, NFAT promotor can be used for auto-regulating the mechanical thresholds by adjusting the MS channel expression. Besides, although the identity of MET channel in hair cells is still unclear, but the method we developed to quantitatively examine the sensibility of Piezo1 channel (Fig. X) is well adapt to all MS channel, such as the Transmembrane channel-like (TMC) channel, which has been thought as a prime contender for the MET channel in hair cells. Even if the nature exists MS channels may not be suitable for repair of hearing disease, the directed evolution platform we developed can be used to extend the audible frequency and intensity of sound that the cells can sense. | ||
{{:Team:SUSTech_Shenzhen/main-content-end}} | {{:Team:SUSTech_Shenzhen/main-content-end}} | ||
{{:Team:SUSTech_Shenzhen/wiki-footer}} | {{:Team:SUSTech_Shenzhen/wiki-footer}} | ||
{{:Team:SUSTech_Shenzhen/themeJs}} | {{:Team:SUSTech_Shenzhen/themeJs}} | ||
Latest revision as of 08:56, 14 November 2016

Demonstrate
Project
Contents
Overview
Precisely controling the mechanical stress applied on cells is an important key to open the door to explore the mechanism of cells' sensation to sound vibration. However, the current research methods used for mechanically activate cells are not pay much concern on the magnitude of mechanical stress. Here we designed a microfluidic chip to quantitatively tune the shear force exerted on cell membrane, and both channel are responsive to such mechical stress. Surprisingly, we discovered that Piezo1-expression CHO cell response to shear force in an biphasic manner and a maximum responsive of ~0.1 pa, which haven't found similar report in the past. We also examined the intracellular calcium response to various combination of frequency and intensity of sound, including both the audible and ultrsound range. We discovered that Piezo1-expression CHO cell can sense the sound that above 15kHz, which indicate the previous identified touching sensing Piezo1 channel could detect sound wave of particular frequency range.
1. Biphasic Response of Piezo1 Channel
We found that Piezo1 channel exhibits two properties: high sensitivity at 0.1Pa, and biphasic response. To the best of our knowledge, both are novel discoveries.2. Acoustic Stimulation
We found that Piezo1 channel can sense acoustic wave from ultrasound to the margin of human audible range. Best response is at 20kHz when using piezo buzzer as sound generator. In microfluidics, the shear stress, about 0.1 pa, apply on cell is continuously dragging Piezo1 channel to open it, however, the mechanism to stop it in higher shear stress is remains unknown. While in acoustic stimulation, the pressure generated by our designed sound wave oscillation is bigger than 100kPa, which is conflict with the result of microfluidics. After consultation with Prof. Bin Tang, who is an expert in biomechanics field. He referred that high frequency oscillation leads visco-elastic transition, in other words, makes cytoskeleton becomes harder, and stiffer cytoskeleton needs lager vibrating force to deform the cytoskeleton.
3. Future Plan
The feedback regulatory motif is most common in the gene regulatory network of cells, including the hair cells. They can change mechanical thresholds by increase or decrease the expression of a mechanosensitive channel (MS) channel. Also, we have proof that downstream GFP of the NFAT (Nuclear factor of activated T-cells) promoter can be successfully induced by long term calcium signal. Thus, NFAT promotor can be used for auto-regulating the mechanical thresholds by adjusting the MS channel expression. Besides, although the identity of MET channel in hair cells is still unclear, but the method we developed to quantitatively examine the sensibility of Piezo1 channel (Fig. X) is well adapt to all MS channel, such as the Transmembrane channel-like (TMC) channel, which has been thought as a prime contender for the MET channel in hair cells. Even if the nature exists MS channels may not be suitable for repair of hearing disease, the directed evolution platform we developed can be used to extend the audible frequency and intensity of sound that the cells can sense.